The use of cffDNA in fetal sex determination during the first trimester of pregnancy of female DMD carriers
- PMID: 25343090
- PMCID: PMC4204568
- DOI: 10.5582/irdr.2012.v1.4.157
The use of cffDNA in fetal sex determination during the first trimester of pregnancy of female DMD carriers
Abstract
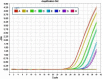
Chorionic villus sampling (CVS) or amniocentesis for fetal sex determination is generally the first step in the prenatal diagnosis of X-linked genetic disorders such as Duchenne muscular dystrophy (DMD). However, non-invasive prenatal diagnostic (NIPD) techniques such as measurement of cell-free fetal DNA (cffDNA) in maternal plasma are preferable given the procedure-related miscarriage rate of CVS. We determined fetal sex during the first trimester using a quantitative real-time polymerase chain reaction (PCR) assay of cffDNA in pregnant carriers of DMD. The fetal sex was confirmed by amniocentesis karyotype analysis and multiplex ligation-dependent probe amplification (MLPA) at 16 weeks. This procedure may avoid unnecessary CVS or amniocentesis of female fetuses.
Keywords: Cell-free fetal DNA (cffDNA); Duchenne muscular dystrophy (DMD); fetal sex determination; non-invasive prenatal diagnostic (NIPD).
Figures
Similar articles
-
Clinical application of fetal sex determination using cell-free fetal DNA in pregnant carriers of X-linked genetic disorders.J Hum Genet. 2011 Apr;56(4):296-9. doi: 10.1038/jhg.2011.7. Epub 2011 Feb 10. J Hum Genet. 2011. PMID: 21307866
-
[Clinical value of MLPA in the prenatal gene diagnosis of Duchenne muscular dystrophy].Zhonghua Fu Chan Ke Za Zhi. 2013 Mar;48(3):161-4. Zhonghua Fu Chan Ke Za Zhi. 2013. PMID: 23849935 Chinese.
-
Non-invasive fetal sex diagnosis in plasma of early weeks pregnants using droplet digital PCR.Mol Med. 2018 Apr 5;24(1):14. doi: 10.1186/s10020-018-0016-7. Mol Med. 2018. PMID: 30134789 Free PMC article.
-
[Prenatal molecular diagnosis of a DMD carrier female fetus by chorionic villus sampling and linkage analysis].Ginecol Obstet Mex. 2009 Feb;77(2):103-9. Ginecol Obstet Mex. 2009. PMID: 19365952 Review. Spanish.
-
Non-invasive prenatal testing for fetal sex determination: is ultrasound still relevant?Eur J Obstet Gynecol Reprod Biol. 2013 Dec;171(2):197-204. doi: 10.1016/j.ejogrb.2013.09.005. Epub 2013 Sep 11. Eur J Obstet Gynecol Reprod Biol. 2013. PMID: 24094458 Review.
Cited by
-
Successful early fetal sex determination using cell-free fetal DNA isolated from maternal capillary blood: A pilot study.Eur J Obstet Gynecol Reprod Biol X. 2019 May 7;3:100038. doi: 10.1016/j.eurox.2019.100038. eCollection 2019 Jul. Eur J Obstet Gynecol Reprod Biol X. 2019. PMID: 31403126 Free PMC article.
-
Three-hour analysis of non-invasive foetal sex determination: application of Plexor chemistry.Hum Genomics. 2016 Apr 4;10:9. doi: 10.1186/s40246-016-0066-2. Hum Genomics. 2016. PMID: 27044517 Free PMC article.
References
-
- Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997; 350:485-487 - PubMed
-
- Lewis C, Hill M, Skirton H, Chitty LS. Fetal sex determination using cell-free fetal DNA: Service users' experiences of and preferences for service delivery. Prenat Diagn. 2012; 32:735-741 - PubMed
-
- Miura K, Higashijima A, Shimada T, Miura S, Yamasaki K, Abe S, Jo O, Kinoshita A, Yoshida A, Yoshimura S, Niikawa N, Yoshiura K, Masuzaki H. Clinical application of fetal sex determination using cell-free fetal DNA in pregnant carriers of X-linked genetic disorders. J Hum Genet. 2011; 56:296-299 - PubMed
-
- Kim SY, Lim JH, Park SY, Kim MY, Choi JS, Ryu HM. Non-invasive prenatal determination of fetal gender using QF-PCR analysis of cell-free fetal DNA in maternal plasma. Clin Chim Acta. 2012; 413:600-604 - PubMed
LinkOut - more resources
Full Text Sources
