Increased adrenergic signaling is responsible for decreased glucose-stimulated insulin secretion in the chronically hyperinsulinemic ovine fetus
- PMID: 25343274
- PMCID: PMC4272391
- DOI: 10.1210/en.2014-1393
Increased adrenergic signaling is responsible for decreased glucose-stimulated insulin secretion in the chronically hyperinsulinemic ovine fetus
Abstract
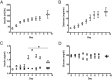
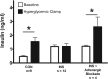
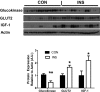
Insulin may stimulate its own insulin secretion and is a potent growth factor for the pancreatic β-cell. Complications of pregnancy, such as diabetes and intrauterine growth restriction, are associated with changes in fetal insulin concentrations, secretion, and β-cell mass. However, glucose concentrations are also abnormal in these conditions. The direct effect of chronic fetal hyperinsulinemia with euglycemia on fetal insulin secretion and β-cell mass has not been tested. We hypothesized that chronic fetal hyperinsulinemia with euglycemia would increase glucose-stimulated insulin secretion (GSIS) and β-cell mass in the ovine fetus. Singleton ovine fetuses were infused with iv insulin to produce high physiological insulin concentrations, or saline for 7-10 days. The hyperinsulinemic animals also received a direct glucose infusion to maintain euglycemia. GSIS, measured at 133 ± 1 days of gestation, was significantly attenuated in the hyperinsulinemic fetuses (P < .05). There was no change in β-cell mass. The hyperinsulinemic fetuses also had decreased oxygen (P < .05) and higher norepinephrine (1160 ± 438 vs 522 ± 106 pg/mL; P < .005). Acute pharmacologic adrenergic blockade restored GSIS in the hyperinsulinemic-euglycemic fetuses, demonstrating that increased adrenergic signaling mediates decreased GSIS in these fetuses.
Figures


References
-
- Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96(3):329–339. - PubMed
-
- Muller D, Jones PM, Persaud SJ. Autocrine anti-apoptotic and proliferative effects of insulin in pancreatic β-cells. FEBS Lett. 2006;580(30):6977–6980. - PubMed
-
- Aspinwall CA, Lakey JR, Kennedy RT. Insulin-stimulated insulin secretion in single pancreatic β cells. J Biol Chem. 1999;274(10):6360–6365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HD060688/HD/NICHD NIH HHS/United States
- UL1TR001082/TR/NCATS NIH HHS/United States
- R03 DK102972/DK/NIDDK NIH HHS/United States
- P30CA046934/CA/NCI NIH HHS/United States
- KL2 TR001080/TR/NCATS NIH HHS/United States
- T32007186-32/PHS HHS/United States
- K01-DK090199/DK/NIDDK NIH HHS/United States
- R01DK088139/DK/NIDDK NIH HHS/United States
- K12 HD068372/HD/NICHD NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- P30DK57516/DK/NIDDK NIH HHS/United States
- R01 DK088139/DK/NIDDK NIH HHS/United States
- P30 DK057516/DK/NIDDK NIH HHS/United States
- TL1 TR001081/TR/NCATS NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- K01 DK090199/DK/NIDDK NIH HHS/United States
- TL1TR001081/TR/NCATS NIH HHS/United States
- K12HD068372/HD/NICHD NIH HHS/United States
- K12 HD057022/HD/NICHD NIH HHS/United States
- KL2TR001080/TR/NCATS NIH HHS/United States
- K08HD060688/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

