Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds
- PMID: 25343343
- PMCID: PMC4410281
- DOI: 10.1089/ten.TEC.2014.0414
Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds
Abstract
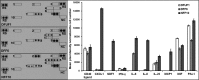
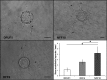
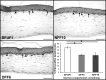
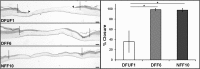
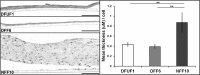
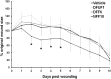
Diabetic foot ulcers (DFU) are a major, debilitating complication of diabetes mellitus. Unfortunately, many DFUs are refractory to existing treatments and frequently lead to amputation. The development of more effective therapies has been hampered by the lack of predictive in vitro methods to investigate the mechanisms underlying impaired healing. To address this need for realistic wound-healing models, we established patient-derived fibroblasts from DFUs and site-matched controls and used them to construct three-dimensional (3D) models of chronic wound healing. Incorporation of DFU-derived fibroblasts into these models accurately recapitulated the following key aspects of chronic ulcers: reduced stimulation of angiogenesis, increased keratinocyte proliferation, decreased re-epithelialization, and impaired extracellular matrix deposition. In addition to reflecting clinical attributes of DFUs, the wound-healing potential of DFU fibroblasts demonstrated in this suite of models correlated with in vivo wound closure in mice. Thus, the reported panel of 3D DFU models provides a more biologically relevant platform for elucidating the cell-cell and cell-matrix-related mechanisms responsible for chronic wound pathogenesis and may improve translation of in vitro findings into efficacious clinical applications.
Figures
References
-
- Boulton A.J.M., Vileikyte L., Ragnarson-Tennvall G., and Apelqvist J.The global burden of diabetic foot disease. Lancet 366,1719, 2005 - PubMed
-
- Breslin S., and O'Driscoll L.Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18,240, 2013 - PubMed
-
- Singer A.J., and Clark R.A.Cutaneous wound healing. N Engl J Med 341,738, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

