Single cell adhesion assay using computer controlled micropipette
- PMID: 25343359
- PMCID: PMC4208850
- DOI: 10.1371/journal.pone.0111450
Single cell adhesion assay using computer controlled micropipette
Abstract
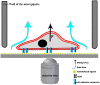
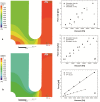
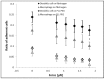
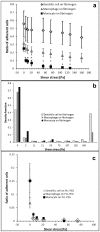
Cell adhesion is a fundamental phenomenon vital for all multicellular organisms. Recognition of and adhesion to specific macromolecules is a crucial task of leukocytes to initiate the immune response. To gain statistically reliable information of cell adhesion, large numbers of cells should be measured. However, direct measurement of the adhesion force of single cells is still challenging and today's techniques typically have an extremely low throughput (5-10 cells per day). Here, we introduce a computer controlled micropipette mounted onto a normal inverted microscope for probing single cell interactions with specific macromolecules. We calculated the estimated hydrodynamic lifting force acting on target cells by the numerical simulation of the flow at the micropipette tip. The adhesion force of surface attached cells could be accurately probed by repeating the pick-up process with increasing vacuum applied in the pipette positioned above the cell under investigation. Using the introduced methodology hundreds of cells adhered to specific macromolecules were measured one by one in a relatively short period of time (∼30 min). We blocked nonspecific cell adhesion by the protein non-adhesive PLL-g-PEG polymer. We found that human primary monocytes are less adherent to fibrinogen than their in vitro differentiated descendants: macrophages and dendritic cells, the latter producing the highest average adhesion force. Validation of the here introduced method was achieved by the hydrostatic step-pressure micropipette manipulation technique. Additionally the result was reinforced in standard microfluidic shear stress channels. Nevertheless, automated micropipette gave higher sensitivity and less side-effect than the shear stress channel. Using our technique, the probed single cells can be easily picked up and further investigated by other techniques; a definite advantage of the computer controlled micropipette. Our experiments revealed the existence of a sub-population of strongly fibrinogen adherent cells appearing in macrophages and highly represented in dendritic cells, but not observed in monocytes.
Conflict of interest statement
Figures



Similar articles
-
Adhesion kinetics of human primary monocytes, dendritic cells, and macrophages: Dynamic cell adhesion measurements with a label-free optical biosensor and their comparison with end-point assays.Biointerphases. 2016 Sep 1;11(3):031001. doi: 10.1116/1.4954789. Biointerphases. 2016. PMID: 27368161
-
Nanonewton scale adhesion force measurements on biotinylated microbeads with a robotic micropipette.J Colloid Interface Sci. 2021 Nov 15;602:291-299. doi: 10.1016/j.jcis.2021.05.180. Epub 2021 Jun 3. J Colloid Interface Sci. 2021. PMID: 34130175
-
Cellular self-organization on micro-structured surfaces.Soft Matter. 2014 Apr 14;10(14):2397-404. doi: 10.1039/c3sm52419a. Soft Matter. 2014. PMID: 24623049
-
Microfluidic technology for investigation of protein function in single adherent cells.Methods Enzymol. 2019;628:145-172. doi: 10.1016/bs.mie.2019.07.038. Epub 2019 Sep 3. Methods Enzymol. 2019. PMID: 31668227 Review.
-
Quantitative methods for analyzing cell-cell adhesion in development.Dev Biol. 2015 May 1;401(1):165-74. doi: 10.1016/j.ydbio.2014.11.002. Epub 2014 Nov 18. Dev Biol. 2015. PMID: 25448695 Review.
Cited by
-
Dissociation Constant of Integrin-RGD Binding in Live Cells from Automated Micropipette and Label-Free Optical Data.Biosensors (Basel). 2021 Jan 24;11(2):32. doi: 10.3390/bios11020032. Biosensors (Basel). 2021. PMID: 33498959 Free PMC article.
-
CD11c/CD18 Dominates Adhesion of Human Monocytes, Macrophages and Dendritic Cells over CD11b/CD18.PLoS One. 2016 Sep 22;11(9):e0163120. doi: 10.1371/journal.pone.0163120. eCollection 2016. PLoS One. 2016. PMID: 27658051 Free PMC article.
-
Differentiation of single lymphoma primary cells and normal B-cells based on their adhesion to mesenchymal stromal cells in optical tweezers.Sci Rep. 2019 Jul 8;9(1):9885. doi: 10.1038/s41598-019-46086-y. Sci Rep. 2019. PMID: 31285461 Free PMC article.
-
A Review of Single-Cell Adhesion Force Kinetics and Applications.Cells. 2021 Mar 5;10(3):577. doi: 10.3390/cells10030577. Cells. 2021. PMID: 33808043 Free PMC article. Review.
-
Single-cell adhesion strength and contact density drops in the M phase of cancer cells.Sci Rep. 2021 Sep 16;11(1):18500. doi: 10.1038/s41598-021-97734-1. Sci Rep. 2021. PMID: 34531409 Free PMC article.
References
-
- Alberts B (1994) Molecular biology of the cell. (3rd Ed) New York: Garland Publishing. 966–996 p.
-
- Kim C, Ye F, Ginsberg MH (2011) Regulation of Integrin Activation. Annual Review of Cell and Developmental Biology 27: 321–345. - PubMed
-
- Hogg N, Patzak I, Willenbrock F (2011) The insider’s guide to leukocyte integrin signalling and function. Nature Reviews Immunology. 11: 416–426. - PubMed
-
- Gahmberg CG (1997) Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Current Opinion in Cell Biology 9: 643–650. - PubMed
-
- Hogg N, Henderson R, Leitinger B, McDowall A, Porter J, et al. (2002) Mechanisms contributing to the activity of integrins on leukocytes. Immunological Reviews 186: 164–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

