Characterization of bovine interferon α1: expression in yeast Pichia pastoris, biological activities, and physicochemical characteristics
- PMID: 25343404
- PMCID: PMC4350256
- DOI: 10.1089/jir.2013.0139
Characterization of bovine interferon α1: expression in yeast Pichia pastoris, biological activities, and physicochemical characteristics
Abstract
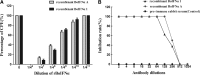
A bovine interferon α (BoIFNα) gene that included signal sequence was amplified from bovine liver genomic DNA. The gene was named BoIFN-α1 according to the position at which the encoded gene of the bovine IFN was located in the bovine genome. The sequence included a 23-amino-acid signal peptide and a 166-amino-acid mature peptide. The structural characteristics and phylogenetic relationships of the BoIFN-α1 gene were analyzed. A recombinant mature BoIFN-α1 (rBoIFN-α1) was expressed in the yeast Pichia pastoris. Physicochemical characteristics and antiviral activity were determined in vitro. Recombinant BoIFN-α1 was found to be highly sensitive to trypsin and stable at pH 2.0 or 65°C. It also exhibited antiviral activity, which was neutralized by a rabbit anti-rBoIFNα polyclonal antibody. This study revealed that rBoIFN-α1 has the typical characteristics of IFNα and can be used for both research and industrial application.
Figures





Similar articles
-
Characterization of the biological activities and physicochemical characteristics of recombinant bovine interferon-α₁₄.Mol Immunol. 2015 Mar;64(1):163-9. doi: 10.1016/j.molimm.2014.11.011. Epub 2014 Dec 3. Mol Immunol. 2015. PMID: 25480392
-
Characterization and antivirus activities of a novel bovine IFN-omega24.Mol Immunol. 2015 Aug;66(2):357-63. doi: 10.1016/j.molimm.2015.04.009. Epub 2015 May 18. Mol Immunol. 2015. PMID: 25951414
-
Construction and characterization of a potent, long-lasting recombinant human serum albumin-interferon α1 fusion protein expressed in Pichia pastoris.Protein Expr Purif. 2013 Aug;90(2):124-8. doi: 10.1016/j.pep.2013.05.002. Epub 2013 Jun 6. Protein Expr Purif. 2013. PMID: 23748141
-
Identification and characterization of a rabbit novel IFN-α unlocated in genome.Dev Comp Immunol. 2018 Jan;78:91-99. doi: 10.1016/j.dci.2017.09.016. Epub 2017 Sep 21. Dev Comp Immunol. 2018. PMID: 28942155
-
Biological Activity of Optimized Codon Bovine Type III Interferon Expressed in Pichia pastoris.Viruses. 2023 Apr 30;15(5):1101. doi: 10.3390/v15051101. Viruses. 2023. PMID: 37243187 Free PMC article.
Cited by
-
Evolutionary Pattern of Interferon Alpha Genes in Bovidae and Genetic Diversity of IFNAA in the Bovine Genome.Front Immunol. 2020 Sep 30;11:580412. doi: 10.3389/fimmu.2020.580412. eCollection 2020. Front Immunol. 2020. PMID: 33117386 Free PMC article.
-
Antiviral activity of mink interferon alpha expressed in the yeast Pichia pastoris.Front Vet Sci. 2022 Sep 14;9:976347. doi: 10.3389/fvets.2022.976347. eCollection 2022. Front Vet Sci. 2022. PMID: 36187832 Free PMC article.
-
Blocking virus infection with BacMam virus delivery bovine interferon-α gene.Virulence. 2024 Dec;15(1):2435372. doi: 10.1080/21505594.2024.2435372. Epub 2024 Nov 29. Virulence. 2024. PMID: 39611624 Free PMC article.
-
Molecular cloning and transcriptional regulation of Indian peafowl (Pavo cristatus) IFN-α gene.Cell Stress Chaperones. 2019 Mar;24(2):323-332. doi: 10.1007/s12192-018-00962-0. Epub 2019 Jan 30. Cell Stress Chaperones. 2019. PMID: 30701479 Free PMC article.
-
Molecular characterization and biological activity of bovine interferon-omega3.Res Vet Sci. 2017 Dec;115:125-131. doi: 10.1016/j.rvsc.2017.01.028. Epub 2017 Feb 10. Res Vet Sci. 2017. PMID: 28254416 Free PMC article.
References
-
- Cao ZW. 2004. An effective method of Tricine-SDS-PAGE for separating the 1 kDa peptide. China Biotechnol 24:74–76
-
- Chaplin PJ, Entrican G, Gelder KI, Collins RA. 1996a. Cloning and biologic activities of a bovine interferon-α isolated from the epithelium of rotavirus-infected calf. J Interferon Cytokine Res 16:25–30 - PubMed
-
- Chaplin PJ, Parsons KR, Collins RA. 1996b. The cloning of cattle interferon-A subtypes isolated from the gut epithelium of rotavirus-infected calves. Immunogenetics 44:143–145 - PubMed
-
- Damasceno LM, Huang CJ, Batt CA. 2012. Protein secretion in Pichia pastoris and advances in protein production. Appl Microbiol Biotechnol 93:31–39 - PubMed
-
- Fish EN, Banejee NK, Stebbing N. 1989. The role of three domains in the biological activity of human IFN-α. J Interferon Res 9:97–114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

