DDX3X induces primary EGFR-TKI resistance based on intratumor heterogeneity in lung cancer cells harboring EGFR-activating mutations
- PMID: 25343452
- PMCID: PMC4208809
- DOI: 10.1371/journal.pone.0111019
DDX3X induces primary EGFR-TKI resistance based on intratumor heterogeneity in lung cancer cells harboring EGFR-activating mutations
Abstract
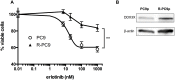
The specific mechanisms how lung cancer cells harboring epidermal growth factor receptor (EGFR) activating mutations can survive treatment with EGFR-tyrosine kinase inhibitors (TKIs) until they eventually acquire treatment-resistance genetic mutations are unclear. The phenotypic diversity of cancer cells caused by genetic or epigenetic alterations (intratumor heterogeneity) confers treatment failure and may foster tumor evolution through Darwinian selection. Recently, we found DDX3X as the protein that was preferentially expressed in murine melanoma with cancer stem cell (CSC)-like phenotypes by proteome analysis. In this study, we transfected PC9, human lung cancer cells harboring EGFR exon19 deletion, with cDNA encoding DDX3X and found that DDX3X, an ATP-dependent RNA helicase, induced CSC-like phenotypes and the epithelial-mesenchymal transition (EMT) accompanied with loss of sensitivity to EGFR-TKI. DDX3X expression was associated with upregulation of Sox2 and increase of cancer cells exhibiting CSC-like phenotypes, such as anchorage-independent proliferation, strong expression of CD44, and aldehyde dehydrogenase (ALDH). The EMT with switching from E-cadherin to N-cadherin was also facilitated by DDX3X. Either ligand-independent or ligand-induced EGFR phosphorylation was inhibited in lung cancer cells that strongly expressed DDX3X. Lack of EGFR signal addiction resulted in resistance to EGFR-TKI. Moreover, we found a small nonadherent subpopulation that strongly expressed DDX3X accompanied by the same stem cell-like properties and the EMT in parental PC9 cells. The unique subpopulation lacked EGFR signaling and was highly resistant to EGFR-TKI. In conclusion, our data indicate that DDX3X may play a critical role for inducing phenotypic diversity, and that treatment targeting DDX3X may overcome primary resistance to EGFR-TKI resulting from intratumor heterogeneity.
Conflict of interest statement
Figures





References
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128. - PubMed
-
- Lahn BT, Page DC (1997) Functional coherence of the human Y chromosome. Science 278: 675–680. - PubMed
-
- Rocak S, Linder P (2004) DEAD-box proteins: the driving forces behind RNA metabolism. Nat Rev Mol Cell Biol 5: 232–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

