Transplantation of heterospheroids of islet cells and mesenchymal stem cells for effective angiogenesis and antiapoptosis
- PMID: 25344077
- PMCID: PMC4356221
- DOI: 10.1089/ten.TEA.2014.0022
Transplantation of heterospheroids of islet cells and mesenchymal stem cells for effective angiogenesis and antiapoptosis
Abstract
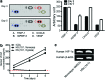
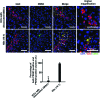
Although islet transplantation has been suggested as an alternative therapy for type 1 diabetes, there are efficiency concerns that are attributed to poor engraftment of transplanted islets. Hypoxic condition and delayed vasculogenesis induce necrosis and apoptosis of the transplanted islets. To overcome these limitations in islet transplantation, heterospheroids (HSs), which consist of rat islet cells (ICs) and human bone marrow-derived mesenchymal stem cells (hMSCs), were transplanted to the kidney and liver. The HSs cultured under the hypoxic condition system exhibited a significant increase in antiapoptotic gene expression in ICs. hMSCs in the HSs secreted angiogenic and antiapoptotic proteins. With the HS system, ICs and hMSCs were successfully located in the same area of the liver after transplantation of HSs through the portal vein, whereas the transplantation of islets and the dissociated hMSCs did not result in localization of transplanted ICs and hMSCs in the same area. HS transplantation resulted in an increase in angiogenesis at the transplantation area and a decrease in the apoptosis of transplanted ICs after transplantation into the kidney subcapsule compared with transplantation of islet cell clusters (ICCs). Insulin production levels of ICs were higher in the HS transplantation group compared with the ICC transplantation group. The HS system may be a more efficient transplantation method than the conventional methods for the treatment of type 1 diabetes.
Figures





References
-
- Shapiro A.M., Ricordi C., Hering B.J., Auchincloss H., Lindblad R., Robertson R.P., et al. . International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355,1318, 2006 - PubMed
-
- Jeong J.H., Hong S.W., Hong S., Yook S., Jung Y., Park J.B., et al. . Surface camouflage of pancreatic islets using 6-arm-PEG-catechol in combined therapy with tacrolimus and anti-CD154 monoclonal antibody for xenotransplantation. Biomaterials 32,7961, 2011 - PubMed
-
- Bennet W., Groth C.G., Larsson R., Nilsson B., and Korsgren O.Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci 105,125, 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

