Selection for growth on 3-nitrotoluene by 2-nitrotoluene-utilizing Acidovorax sp. strain JS42 identifies nitroarene dioxygenases with altered specificities
- PMID: 25344236
- PMCID: PMC4272708
- DOI: 10.1128/AEM.02772-14
Selection for growth on 3-nitrotoluene by 2-nitrotoluene-utilizing Acidovorax sp. strain JS42 identifies nitroarene dioxygenases with altered specificities
Abstract
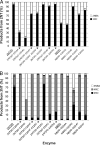
Acidovorax sp. strain JS42 uses 2-nitrotoluene as a sole source of carbon and energy. The first enzyme of the degradation pathway, 2-nitrotoluene 2,3-dioxygenase, adds both atoms of molecular oxygen to 2-nitrotoluene, forming nitrite and 3-methylcatechol. All three mononitrotoluene isomers serve as substrates for 2-nitrotoluene dioxygenase, but strain JS42 is unable to grow on 3- or 4-nitrotoluene. Using both long- and short-term selections, we obtained spontaneous mutants of strain JS42 that grew on 3-nitrotoluene. All of the strains obtained by short-term selection had mutations in the gene encoding the α subunit of 2-nitrotoluene dioxygenase that changed isoleucine 204 at the active site to valine. Those strains obtained by long-term selections had mutations that changed the same residue to valine, alanine, or threonine or changed the alanine at position 405, which is just outside the active site, to glycine. All of these changes altered the regiospecificity of the enzymes with 3-nitrotoluene such that 4-methylcatechol was the primary product rather than 3-methylcatechol. Kinetic analyses indicated that the evolved enzymes had enhanced affinities for 3-nitrotoluene and were more catalytically efficient with 3-nitrotoluene than the wild-type enzyme. In contrast, the corresponding amino acid substitutions in the closely related enzyme nitrobenzene 1,2-dioxygenase were detrimental to enzyme activity. When cloned genes encoding the evolved dioxygenases were introduced into a JS42 mutant lacking a functional dioxygenase, the strains acquired the ability to grow on 3-nitrotoluene but with significantly longer doubling times than the evolved strains, suggesting that additional beneficial mutations occurred elsewhere in the genome.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Hartter DR. 1985. The use and importance of nitroaromatic chemicals in the chemical industry, p 1–14. In Rickert DE. (ed), Toxicity of nitroaromatic compounds. Chemical Industry Institute of Toxicology series. Hemisphere, Washington, DC.
-
- Swoboda-Colberg NG. 1995. Chemical contamination of the environment: sources, types, and fate of synthetic organic chemicals, p 27–74. In Young LY, Cerniglia CE (ed), Microbial transformation and degradation of toxic organic chemicals. Wiley-Liss, New York, NY.
-
- Burmaster DE. 1982. The new pollution: groundwater contamination. Environment 24:6–13, 22–36.
-
- Callahan MA, Slimak MW, Gabel NW, May JP, Fowler CF, Freed JR, Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, Gould C. 1979. Water-related environmental fate of 129 priority pollutants. EPA, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

