Salivary mucins protect surfaces from colonization by cariogenic bacteria
- PMID: 25344244
- PMCID: PMC4272720
- DOI: 10.1128/AEM.02573-14
Salivary mucins protect surfaces from colonization by cariogenic bacteria
Abstract
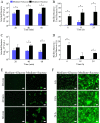
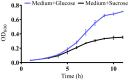
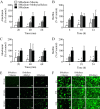
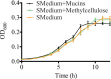
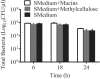
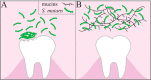
Understanding how the body's natural defenses function to protect the oral cavity from the myriad of bacteria that colonize its surfaces is an ongoing topic of research that can lead to breakthroughs in treatment and prevention. One key defense mechanism on all moist epithelial linings, such as the mouth, gastrointestinal tract, and lungs, is a layer of thick, well-hydrated mucus. The main gel-forming components of mucus are mucins, large glycoproteins that play a key role in host defense. This study focuses on elucidating the connection between MUC5B salivary mucins and dental caries, one of the most common oral diseases. Dental caries is predominantly caused by Streptococcus mutans attachment and biofilm formation on the tooth surface. Once S. mutans attaches to the tooth, it produces organic acids as metabolic by-products that dissolve tooth enamel, leading to cavity formation. We utilize CFU counts and fluorescence microscopy to quantitatively show that S. mutans attachment and biofilm formation are most robust in the presence of sucrose and that aqueous solutions of purified human MUC5B protect surfaces by acting as an antibiofouling agent in the presence of sucrose. In addition, we find that MUC5B does not alter S. mutans growth and decreases surface attachment and biofilm formation by maintaining S. mutans in the planktonic form. These insights point to the importance of salivary mucins in oral health and lead to a better understanding of how MUC5B could play a role in cavity prevention or diagnosis.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Brockhausen I, Schachter H, Stanley P. 2009. O-GalNAc glycans. In Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, Etzler M (ed), Essentials of glycobiology, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. - PubMed
-
- Perez-Vilar J, Mabolo R. 2007. Gel-forming mucins. Notions from in vitro studies. Histol Histopathol 22:455–464. - PubMed
-
- Van der Sluis M, De Koning B, De Bruijn A, Velcich A, Meijerink J, Van Goudoever J, Büller H, Dekker J, Van Seuningen I, Renes I, Einerhand A. 2006. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131:117–129. doi:10.1053/j.gastro.2006.04.020. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

