Synaptotagmin-7 links fusion-activated Ca²⁺ entry and fusion pore dilation
- PMID: 25344253
- PMCID: PMC4265738
- DOI: 10.1242/jcs.153742
Synaptotagmin-7 links fusion-activated Ca²⁺ entry and fusion pore dilation
Abstract
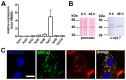
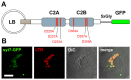
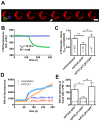
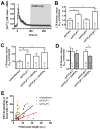
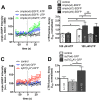
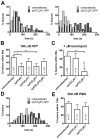
Ca(2+)-dependent regulation of fusion pore dilation and closure is a key mechanism determining the output of cellular secretion. We have recently described 'fusion-activated' Ca(2+) entry (FACE) following exocytosis of lamellar bodies in alveolar type II cells. FACE regulates fusion pore expansion and facilitates secretion. However, the mechanisms linking this locally restricted Ca(2+) signal and fusion pore expansion were still elusive. Here, we demonstrate that synaptotagmin-7 (Syt7) is expressed on lamellar bodies and links FACE and fusion pore dilation. We directly assessed dynamic changes in fusion pore diameters by analysing diffusion of fluorophores across fusion pores. Expressing wild-type Syt7 or a mutant Syt7 with impaired Ca(2+)-binding to the C2 domains revealed that binding of Ca(2+) to the C2A domain facilitates FACE-induced pore dilation, probably by inhibiting translocation of complexin-2 to fused vesicles. However, the C2A domain hampered Ca(2+)-dependent exocytosis of lamellar bodies. These findings support the hypothesis that Syt7 modulates fusion pore expansion in large secretory organelles and extend our picture that lamellar bodies contain the necessary molecular inventory to facilitate secretion during the exocytic post-fusion phase. Moreover, regulating Syt7 levels on lamellar bodies appears to be essential in order that exocytosis is not impeded during the pre-fusion phase.
Keywords: Calcium; Exocytosis; FACE; Fusion pore; Lamellar body; Synaptotagmin.
© 2014. Published by The Company of Biologists Ltd.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

