LQT1 mutations in KCNQ1 C-terminus assembly domain suppress IKs using different mechanisms
- PMID: 25344363
- PMCID: PMC4296111
- DOI: 10.1093/cvr/cvu231
LQT1 mutations in KCNQ1 C-terminus assembly domain suppress IKs using different mechanisms
Abstract
Aims: Long QT syndrome 1 (LQT1) mutations in KCNQ1 that decrease cardiac IKs (slowly activating delayed rectifier K(+) current) underlie ventricular arrhythmias and sudden death. LQT1 mutations may suppress IKs by preventing KCNQ1 assembly, disrupting surface trafficking, or inhibiting gating. We investigated mechanisms underlying how three LQT1 mutations in KCNQ1 C-terminus assembly domain (R555H/G589D/L619M) decrease IKs in heterologous cells and cardiomyocytes.
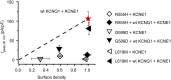
Methods and results: In Chinese hamster ovary (CHO) cells, mutant KCNQ1 + KCNE1 channels either produced no currents (G589D/L619M) or displayed markedly reduced IKs with a right-shifted voltage-dependence of activation (R555H). When co-expressed with wild-type (wt) KCNQ1, the mutant KCNQ1s displayed varying intrinsic dominant-negative capacities that were affected by auxiliary KCNE1. All three mutant KCNQ1s assembled with wt KCNQ1 as determined by fluorescence resonance energy transfer (FRET). We developed an optical quantum dot labelling assay to measure channel surface density. G589D/R555H displayed substantial reductions in surface density, which were either partially (G589D) or fully (R555H) rescued by wt KCNQ1. Unexpectedly, L619M showed no trafficking defect. In adult rat cardiomyocytes, adenovirus-expressed homotetrameric G589D/L619M + KCNE1 channels yielded no currents, whereas R555H + KCNE1 produced diminished IKs with a right-shifted voltage-dependence of activation, mimicking observations in CHO cells. In contrast to heterologous cells, homotetrameric R555H channels showed no trafficking defect in cardiomyocytes.
Conclusion: Distinct LQT1 mutations in KCNQ1 assembly domain decrease IKs using unique combinations of biophysical and trafficking mechanisms. Functional deficits in IKs observed in heterologous cells are mostly, but not completely, recapitulated in adult rat cardiomyocytes. A 'methodological chain' combining approaches in heterologous cells and cardiomyocytes provides mechanistic insights that may help advance personalized therapy for LQT1 mutations.
Keywords: Cardiac myocyte; Channel trafficking; Ion channel; KCNQ1; Long QT syndrome.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2014. For permissions please email: journals.permissions@oup.com.
Figures





References
-
- Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. - PubMed
-
- Charpentier F, Merot J, Loussouarn G, Baro I. Delayed rectifier K(+) currents and cardiac repolarization. J Mol Cell Cardiol. 2010;48:37–44. - PubMed
-
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

