Eligibility determination for clinical trials: development of a case review process at a chiropractic research center
- PMID: 25344427
- PMCID: PMC4221721
- DOI: 10.1186/1745-6215-15-406
Eligibility determination for clinical trials: development of a case review process at a chiropractic research center
Abstract
Background: Systematic procedures addressing the limitations of eligibility determination are needed to improve the quality of participant recruitment and enrollment in randomized clinical trials. This paper describes an eligibility determination process developed by and in use at a chiropractic research center engaged in community recruitment for clinical trials studying spinal pain conditions.
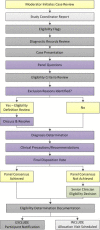
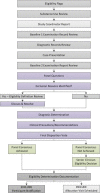
Methods: A team of investigators developed a case review process for application across clinical trials involving chiropractic care. Study personnel representing key study roles including research clinicians, study coordinators, a project manager, and at least one investigator convene in person to determine eligibility for participants following baseline study visit examinations. The research clinician who performed the eligibility examination presents the case and a moderator leads the case review panel through a structured discussion including diagnosis, eligibility criteria, definition review, and clinical precautions. Panel members provide clinical recommendations and determine final eligibility using a structured and moderated voting process.
Results: Through the case review process for three externally funded clinical trials for participants with neck and low back pain, we presented 697 cases, rendering 472 participants eligible for enrollment and excluding 225 individuals. The most common reasons for case review exclusions across the three trials included neck or back pain not meeting diagnostic classifications, safety concerns related to treatment or testing, referral for further evaluation or treatment, and compliance concerns.
Conclusions: The case review process uses the expertise of study coordinators, research clinicians, project managers, and investigators to render eligibility decisions consistent with study aims for the duration of the trial. This formal eligibility determination process includes steps designed to mitigate the potential for participant misclassification from clinician advocacy or misunderstanding of eligibility criteria, and helps ensure that participants can safely take part in study procedures.
Trial registration: The three trials discussed in this article were registered in ClinicalTrials.gov with the ID numbers of NCT00830596 (27 January 2009), NCT01312233 (04 March 2011), and NCT01765751 (30 May 2012).
Figures


References
-
- Macias WL, Vallet B, Bernard GR, Vincent JL, Laterre PF, Nelson DR, Derchak PA, Dhainaut JF. Sources of variability on the estimate of treatment effect in the PROWESS trial: implications for the design and conduct of future studies in severe sepsis. Crit Care Med. 2004;32:2385–2391. doi: 10.1097/01.CCM.0000147440.71142.AC. - DOI - PubMed
-
- Kasai T, Ohe Y, Nishio K, Kunitoh H, Tamura T, Sekine I, Kubota K, Yamamoto N, Nakamura Y, Shinkai T, Kodama T, Saijo N. Factors that influence the eligibility of cases for inclusion in clinical trials. The Lung Cancer Chemotherapy Study Group of the Japan Clinical Oncology Group. Jpn J Clin Oncol. 1998;28:214–221. doi: 10.1093/jjco/28.3.214. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

