Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach
- PMID: 25344439
- PMCID: PMC4287285
- DOI: 10.1208/s12248-014-9674-8
Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach
Abstract
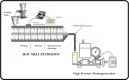
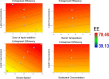
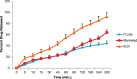
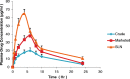
This contribution describes a continuous process for the production of solid lipid nanoparticles (SLN) as drug-carrier systems via hot-melt extrusion (HME). Presently, HME technology has not been used for the manufacturing of SLN. Generally, SLN are prepared as a batch process, which is time consuming and may result in variability of end-product quality attributes. In this study, using Quality by Design (QbD) principles, we were able to achieve continuous production of SLN by combining two processes: HME technology for melt-emulsification and high-pressure homogenization (HPH) for size reduction. Fenofibrate (FBT), a poorly water-soluble model drug, was incorporated into SLN using HME-HPH methods. The developed novel platform demonstrated better process control and size reduction compared to the conventional process of hot homogenization (batch process). Varying the process parameters enabled the production of SLN below 200 nm. The dissolution profile of the FBT SLN prepared by the novel HME-HPH method was faster than that of the crude FBT and a micronized marketed FBT formulation. At the end of a 5-h in vitro dissolution study, a SLN formulation released 92-93% of drug, whereas drug release was approximately 65 and 45% for the marketed micronized formulation and crude drug, respectively. Also, pharmacokinetic study results demonstrated a statistical increase in Cmax, Tmax, and AUC0-24 h in the rate of drug absorption from SLN formulations as compared to the crude drug and marketed micronized formulation. In summary, the present study demonstrated the potential use of hot-melt extrusion technology for continuous and large-scale production of SLN.
Figures


References
-
- Basalious EB, El-Sebaie W, El-Gazayerly O. Application of pharmaceutical QbD for enhancement of the solubility and dissolution of a class II BCS drug using polymeric surfactants and crystallization inhibitors: development of controlled-release tablets. AAPS Pharm Sci Technol. 2011;12:799–810. doi: 10.1208/s12249-011-9646-6. - DOI - PMC - PubMed
-
- US FDA Guidance for industry: PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. Maryland: Silver Spring; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

