Do peripheral and/or central chemoreflexes influence skin blood flow in humans?
- PMID: 25344478
- PMCID: PMC4254106
- DOI: 10.14814/phy2.12181
Do peripheral and/or central chemoreflexes influence skin blood flow in humans?
Abstract
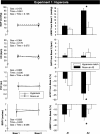
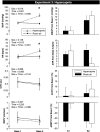
Voluntary apnea activates the central and peripheral chemoreceptors, leading to a rise in sympathetic nerve activity and limb vasoconstriction (i.e., brachial blood flow velocity and forearm cutaneous vascular conductance decrease to a similar extent). Whether peripheral and/or central chemoreceptors contribute to the cutaneous vasoconstrictor response remains unknown. We performed three separate experiments in healthy young men to test the following three hypotheses. First, inhibition of peripheral chemoreceptors with brief hyperoxia inhalation (100% O2) would attenuate the cutaneous vasoconstrictor response to voluntary apnea. Second, activation of the peripheral chemoreceptors with 5 min of hypoxia (10% O2, 90% N2) would augment the cutaneous vasoconstrictor response to voluntary apnea. Third, activation of the central chemoreceptors with 5 min of hypercapnia (7% CO2, 30% O2, 63% N2) would have no influence on cutaneous responses to voluntary apnea. Studies were performed in the supine posture with skin temperature maintained at thermoneutral levels. Beat-by-beat blood pressure, heart rate, brachial blood flow velocity, and cutaneous vascular conductance were measured and changes from baseline were compared between treatments. Relative to room air, hyperoxia attenuated the vasoconstrictor response to voluntary apnea in both muscle (-16 ± 10 vs. -40 ± 12%, P = 0.023) and skin (-14 ± 6 vs. -24 ± 5%, P = 0.033). Neither hypoxia nor hypercapnia had significant effects on cutaneous responses to apnea. These data indicate that skin blood flow is controlled by the peripheral chemoreceptors but not the central chemoreceptors.
Keywords: Blood pressure; chemoreceptors; cutaneous vasoconstriction; heart rate; muscle blood flow; sympathetic activation.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures


Similar articles
-
Sleep-Disordered Breathing Exacerbates Muscle Vasoconstriction and Sympathetic Neural Activation in Patients with Systolic Heart Failure.Circ Heart Fail. 2016 Nov;9(11):e003065. doi: 10.1161/CIRCHEARTFAILURE.116.003065. Circ Heart Fail. 2016. PMID: 28029639
-
Hypoxia augments apnea-induced peripheral vasoconstriction in humans.J Appl Physiol (1985). 2001 Apr;90(4):1516-22. doi: 10.1152/jappl.2001.90.4.1516. J Appl Physiol (1985). 2001. PMID: 11247954
-
Sex differences in forearm vasoconstrictor response to voluntary apnea.Am J Physiol Heart Circ Physiol. 2014 Feb;306(3):H309-16. doi: 10.1152/ajpheart.00746.2013. Epub 2013 Dec 6. Am J Physiol Heart Circ Physiol. 2014. PMID: 24322616 Free PMC article.
-
Chemoreflexes--physiology and clinical implications.Acta Physiol Scand. 2003 Mar;177(3):377-84. doi: 10.1046/j.1365-201X.2003.01083.x. Acta Physiol Scand. 2003. PMID: 12609009 Review.
-
Differential activation of sympathetic discharge to skin and skeletal muscle in humans.Acta Physiol Scand Suppl. 1997;639:1-32. Acta Physiol Scand Suppl. 1997. PMID: 9421582 Review.
References
-
- Bishop C. C., Powell S., Rutt D., Browse N. L. 1986. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke; 17:913-915. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

