How innovative are new drugs launched in the UK? A retrospective study of new drugs listed in the British National Formulary (BNF) 2001-2012
- PMID: 25344485
- PMCID: PMC4212185
- DOI: 10.1136/bmjopen-2014-006235
How innovative are new drugs launched in the UK? A retrospective study of new drugs listed in the British National Formulary (BNF) 2001-2012
Abstract
Objectives: Innovative new drugs offer potential benefits to patients, healthcare systems, governments and the pharmaceutical industry. Recent data suggest annual numbers of new drugs launched in the UK have increased in recent years, and we sought to understand whether this represents increasing numbers of highly innovative drugs being made available or the introduction of increasing numbers of drugs with limited additional therapeutic value.
Design and setting: Retrospective observational study of new drug entries in the British National Formulary (BNF).
Primary and secondary outcome measures: Number of new drugs launched in the UK each year (based on first appearance in the BNF) from 2001 to 2012, including new chemical entities and new biological drugs, categorised by degree of innovativeness according to published criteria that incorporate both clinical usefulness and the nature of the innovation.
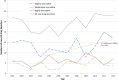
Results: Highly innovative, moderately innovative and slightly innovative drugs made up 26%, 18% and 56% of all newly launched drugs, respectively, for the study period (n=290). There was an upward trend in annual numbers of slightly innovative drugs from 2004 onwards (R(2)=0.44), which aligned closely with the recovery in total numbers of new drugs launched each year since that time. There were no discernible time trends in the highly or moderately innovative categories. New drugs for malignancy and skin disease were most likely to be characterised as highly innovative (44% and 57%, respectively).
Conclusions: Highly innovative new drugs comprise only around a quarter of all new drug launches in the UK. In contrast, drugs categorised as only slightly innovative comprised well over half of all new drugs and annual numbers in this category are increasing. Current policy initiatives that seek to increase the supply of innovative new drugs have long-lead times to impact, and will need careful assessment to ensure they deliver their aims without unintended consequences.
Keywords: Innovation; New drug launches; Pharmaceuticals; United Kingdom; time trends.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Similar articles
-
Decline in new drug launches: myth or reality? Retrospective observational study using 30 years of data from the UK.BMJ Open. 2013 Feb 20;3(2):e002088. doi: 10.1136/bmjopen-2012-002088. Print 2013. BMJ Open. 2013. PMID: 23427198 Free PMC article.
-
Should financial incentives be used to differentially reward 'me-too' and innovative drugs?Pharmacoeconomics. 2010;28(1):1-17. doi: 10.2165/11318770-000000000-00000. Pharmacoeconomics. 2010. PMID: 20014872 Review.
-
Trends in clinical development timeframes for antiviral drugs launched in the UK, 1981-2014: a retrospective observational study.BMJ Open. 2015 Nov 16;5(11):e009333. doi: 10.1136/bmjopen-2015-009333. BMJ Open. 2015. PMID: 26576812 Free PMC article.
-
Provision of medicines information: the example of the British National Formulary.Br J Clin Pharmacol. 2012 Jun;73(6):934-8. doi: 10.1111/j.1365-2125.2012.04241.x. Br J Clin Pharmacol. 2012. PMID: 22360536 Free PMC article.
-
Keynote review: Is declining innovation in the pharmaceutical industry a myth?Drug Discov Today. 2005 Aug 1;10(15):1031-9. doi: 10.1016/S1359-6446(05)03524-5. Drug Discov Today. 2005. PMID: 16055019 Review.
Cited by
-
Genomics for All: International Open Science Genomics Projects and Capacity Building in the Developing World.Front Genet. 2019 Feb 15;10:95. doi: 10.3389/fgene.2019.00095. eCollection 2019. Front Genet. 2019. PMID: 30828348 Free PMC article.
-
Overcoming the Declining Trends in Innovation and Investment in Cardiovascular Therapeutics: Beyond EROOM's Law.JACC Basic Transl Sci. 2017 Oct 30;2(5):613-625. doi: 10.1016/j.jacbts.2017.09.002. eCollection 2017 Oct. JACC Basic Transl Sci. 2017. PMID: 30062175 Free PMC article.
-
Fear of missing out: Drug availability in the United States vs Canada.J Manag Care Spec Pharm. 2024 Dec;30(12):1349-1354. doi: 10.18553/jmcp.2024.30.12.1349. J Manag Care Spec Pharm. 2024. PMID: 39612252 Free PMC article.
-
Adaptive Pathways: Possible Next Steps for Payers in Preparation for Their Potential Implementation.Front Pharmacol. 2017 Aug 23;8:497. doi: 10.3389/fphar.2017.00497. eCollection 2017. Front Pharmacol. 2017. PMID: 28878667 Free PMC article. Review.
-
Trends in the costs of drugs launched in the UK between 1981 and 2015: an analysis of the launch price of drugs in five disease areas.BMJ Open. 2019 May 5;9(5):e027625. doi: 10.1136/bmjopen-2018-027625. BMJ Open. 2019. PMID: 31061053 Free PMC article.
References
-
- Aronson JK, Ferner RE, Hughes DA. Defining rewardable innovation in drug therapy. Nat Rev Drug Discov 2012;11:253–4 - PubMed
-
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ 2003;22:151–85 - PubMed
-
- US Food and Drug Administration (FDA). Innovation or stagnation: challenge and opportunity on the critical path to new medical products. 2004. http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/... (accessed 2 Feb 2012)
-
- Cohen FJ. Macro trends in pharmaceutical innovation. Nat Rev Drug Discov 2005;4:78–84 - PubMed
-
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 2009;8:959–68 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources