Treatment crossovers in time-to-event non-inferiority randomised trials of radiotherapy in patients with breast cancer
- PMID: 25344487
- PMCID: PMC4212183
- DOI: 10.1136/bmjopen-2014-006531
Treatment crossovers in time-to-event non-inferiority randomised trials of radiotherapy in patients with breast cancer
Abstract
Background: In non-inferiority trials of radiotherapy in patients with early stage breast cancer, it is inevitable that some patients will cross over from the experimental arm to the standard arm prior to initiation of any treatment due to complexities in treatment planning or subject preference. Although the intention-to-treat (ITT) analysis is the preferred approach for superiority trials, its role in non-inferiority trials is still under debate. This has led to the use of alternative approaches such as the per-protocol (PP) analysis or the as-treated (AT) analysis, despite the inherent biases of such approaches.
Methods: Using simulations, we investigate the effect of 2%, 5% and 10% random and non-random crossovers prior to radiotherapy initiation on the ITT, PP, AT and the combination of ITT and PP analyses with respect to type I error in trials with time-to-event outcomes. We also evaluate bias and SE of the estimates from the ITT, PP and AT approaches.
Results: The AT approach had the best performance in terms of type I error, but was anticonservative as non-random crossover increased. The ITT and PP approaches were anticonservative under all percentages of random and non-random crossover. Similarly, lowest bias was seen with the AT approach; however, bias increased as the percentage of non-random crossover increased. The ITT and PP had poor performance in terms of bias as crossovers increased.
Conclusions: If minimal crossovers were to occur, we have shown that the AT approach has the lowest type I error rates and smallest opportunity for bias. Results of trials with a high number of crossovers should be interpreted with caution, especially when crossover is non-random. Attempts to prevent crossovers should be maximised.
Keywords: STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
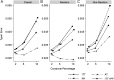
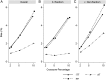
References
-
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513–20 - PubMed
-
- Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol 2013;31:4038–45 - PubMed
-
- D'Agostino RB Sr, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues—the encounters of academic consultants in statistics. Stat Med 2003;22:169–86 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
