Direct binding of arsenic trioxide to AMPK and generation of inhibitory effects on acute myeloid leukemia precursors
- PMID: 25344585
- PMCID: PMC4297272
- DOI: 10.1158/1535-7163.MCT-14-0665-T
Direct binding of arsenic trioxide to AMPK and generation of inhibitory effects on acute myeloid leukemia precursors
Abstract
Arsenic trioxide (As2O3) exhibits potent antineoplastic effects and is used extensively in clinical oncology for the treatment of a subset of patients with acute myeloid leukemia (AML). Although As2O3 is known to regulate activation of several signaling cascades, the key events, accounting for its antileukemic properties, remain to be defined. We provide evidence that arsenic can directly bind to cysteine 299 in AMPKα and inhibit its activity. This inhibition of AMPK by arsenic is required in part for its cytotoxic effects on primitive leukemic progenitors from patients with AML, while concomitant treatment with an AMPK activator antagonizes in vivo the arsenic-induced antileukemic effects in a xenograft AML mouse model. A consequence of AMPK inhibition is activation of the mTOR pathway as a negative regulatory feedback loop. However, when AMPK expression is lost, arsenic-dependent activation of the kinase RSK downstream of MAPK activity compensates the generation of regulatory feedback signals through phosphorylation of downstream mTOR targets. Thus, therapeutic regimens with As2O3 will need to include inhibitors of both the mTOR and RSK pathways in combination to prevent engagement of negative feedback loops and maximize antineoplastic responses.
©2014 American Association for Cancer Research.
Figures

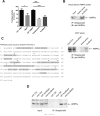
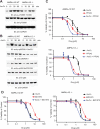
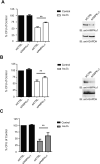
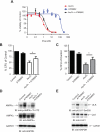

References
-
- Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001;6(Suppl 2):3–10. - PubMed
-
- Cohen MH, Hirschfeld S, Flamm Honig S, Ibrahim A, Johnson JR, O'Leary JJ, et al. Drug approval summaries: arsenic trioxide, tamoxifen citrate, anastrazole, paclitaxel, bexarotene. Oncologist. 2001;6:4–11. - PubMed
-
- Dilda PJ, Hogg PJ. Arsenical-based cancer drugs. Cancer Treat Rev. 2007;33:542–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA121192/CA/NCI NIH HHS/United States
- F32CA183536/CA/NCI NIH HHS/United States
- R01 CA077816/CA/NCI NIH HHS/United States
- I01 CX000916/CX/CSRD VA/United States
- F32 CA183536/CA/NCI NIH HHS/United States
- R01 CA155566/CA/NCI NIH HHS/United States
- CA77816/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- U01 CA151461/CA/NCI NIH HHS/United States
- T32CA070085/CA/NCI NIH HHS/United States
- T32 CA070085/CA/NCI NIH HHS/United States
- CA155566/CA/NCI NIH HHS/United States
- CA121192/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

