E2F transcription factor 1 regulates cellular and organismal senescence by inhibiting Forkhead box O transcription factors
- PMID: 25344604
- PMCID: PMC4256352
- DOI: 10.1074/jbc.M114.587170
E2F transcription factor 1 regulates cellular and organismal senescence by inhibiting Forkhead box O transcription factors
Abstract
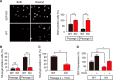
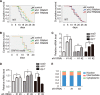
E2F1 and FOXO3 are two transcription factors that have been shown to participate in cellular senescence. Previous report reveals that E2F1 enhanced cellular senescence in human fibroblast cells, while FOXO transcription factors play against senescence by regulation reactive oxygen species scavenging proteins. However, their functional interplay has been unclear. Here we use E2F1 knock-out murine Embryonic fibroblasts (MEFs), knockdown RNAi constructs, and ectopic expression of E2F1 to show that it functions by negatively regulating FOXO3. E2F1 attenuates FOXO3-mediated expression of MnSOD and Catalase without affecting FOXO3 protein stability, subcellular localization, or phosphorylation by Akt. We mapped the interaction between E2F1 and FOXO3 to a region including the DNA binding domain of E2F1 and the C-terminal transcription-activation domain of FOXO3. We propose that E2F1 inhibits FOXO3-dependent transcription by directly binding FOXO3 in the nucleus and preventing activation of its target genes. Moreover, knockdown of the Caenorhabditis elegans E2F1 ortholog efl-1 significantly extends lifespan in a manner that requires the activity of the C. elegans FOXO gene daf-16. We conclude that there is an evolutionarily conserved signaling connection between E2F1 and FOXO3, which regulates cellular senescence and aging by regulating the activity of FOXO3. We speculate that drugs and/or therapies that inhibit this physical interaction might be good candidates for reducing cellular senescence and increasing longevity.
Keywords: E2F Transcription Factor; FOXO; Oxidative Stress; Senescence; Transcription Regulation.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Wu Z., Zheng S., Yu Q. (2009) The E2F family and the role of E2F1 in apoptosis. Int. J. Biochem. Cell Biol. 41, 2389–2397 - PubMed
-
- Matsumura I., Tanaka H., Kanakura Y. (2003) E2F1 and c-Myc in cell growth and death. Cell Cycle 2, 333–338 - PubMed
-
- Field S. J., Tsai F. Y., Kuo F., Zubiaga A. M., Kaelin W. G., Jr., Livingston D. M., Orkin S. H., Greenberg M. E. (1996) E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell 85, 549–561 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

