Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy
- PMID: 25344692
- PMCID: PMC4676084
- DOI: 10.1038/ng.3122
Mutations in PLK4, encoding a master regulator of centriole biogenesis, cause microcephaly, growth failure and retinopathy
Abstract
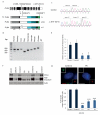
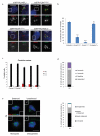
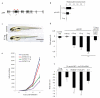
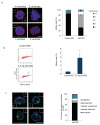
Centrioles are essential for ciliogenesis. However, mutations in centriole biogenesis genes have been reported in primary microcephaly and Seckel syndrome, disorders without the hallmark clinical features of ciliopathies. Here we identify mutations in the genes encoding PLK4 kinase, a master regulator of centriole duplication, and its substrate TUBGCP6 in individuals with microcephalic primordial dwarfism and additional congenital anomalies, including retinopathy, thereby extending the human phenotypic spectrum associated with centriole dysfunction. Furthermore, we establish that different levels of impaired PLK4 activity result in growth and cilia phenotypes, providing a mechanism by which microcephaly disorders can occur with or without ciliopathic features.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

