Extracellular matrix components in the pathogenesis of type 1 diabetes
- PMID: 25344787
- PMCID: PMC4238291
- DOI: 10.1007/s11892-014-0552-7
Extracellular matrix components in the pathogenesis of type 1 diabetes
Abstract
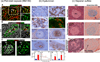
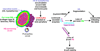
Type 1 diabetes (T1D) results from progressive immune cell-mediated destruction of pancreatic β cells. As immune cells migrate into the islets, they pass through the extracellular matrix (ECM). This ECM is composed of different macromolecules localized to different compartments within and surrounding islets; however, the involvement of this ECM in the development of human T1D is not well understood. Here, we summarize our recent findings from human and mouse studies illustrating how specific components of the islet ECM that constitute basement membranes and interstitial matrix of the islets, and surprisingly, the intracellular composition of islet β cells themselves, are significantly altered during the pathogenesis of T1D. Our focus is on the ECM molecules laminins, collagens, heparan sulfate/heparan sulfate proteoglycans, and hyaluronan, as well as on the enzymes that degrade these ECM components. We propose that islet and lymphoid tissue ECM composition and organization are critical to promoting immune cell activation, islet invasion, and destruction of islet β cells in T1D.
Figures
References
-
-
Hynes RO, Yamada KM, editors. Extracellular Matrix Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2012. Excellent up-to-date coverage of different ECM components.
-
-
-
Karamanos N, editor. Extracellular Matrix: Pathobiology and Signaling. Berlin/Boston: Walter de Gruyter; 2012. Outstanding collection of chapters covering information on how different components of ECM impact cellular signaling and cell phenotype.
-
-
- Mecham RP, editor. Biology of the Extracellular Matrix. Berlin: Springer-Verlag; 2011. The Extracellular Matrix: an Overview.
-
- Hay ED. Cell biology of extracellular matrix. New York: Plenum Press; 1991.
-
- Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

