Axl as a mediator of cellular growth and survival
- PMID: 25344858
- PMCID: PMC4253401
- DOI: 10.18632/oncotarget.2422
Axl as a mediator of cellular growth and survival
Abstract
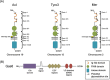
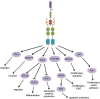
The control of cellular growth and proliferation is key to the maintenance of homeostasis. Survival, proliferation, and arrest are regulated, in part, by Growth Arrest Specific 6 (Gas6) through binding to members of the TAM receptor tyrosine kinase family. Activation of the TAM receptors leads to downstream signaling through common kinases, but the exact mechanism within each cellular context varies and remains to be completely elucidated. Deregulation of the TAM family, due to its central role in mediating cellular proliferation, has been implicated in multiple diseases. Axl was cloned as the first TAM receptor in a search for genes involved in the progression of chronic to acute-phase leukemia, and has since been established as playing a critical role in the progression of cancer. The oncogenic nature of Axl is demonstrated through its activation of signaling pathways involved in proliferation, migration, inhibition of apoptosis, and therapeutic resistance. Despite its recent discovery, significant progress has been made in the development of effective clinical therapeutics targeting Axl. In order to accurately define the role of Axl in normal and diseased processes, it must be analyzed in a cell type-specific context.
Conflict of interest statement
Nothing to declare.
Figures



References
-
- Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1994;5:647–657. - PubMed
-
- Ohashi K, Mizuno K, Kuma K, Miyata T, Nakamura T. Cloning of the cDNA for a novel receptor tyrosine kinase, Sky, predominantly expressed in brain. Oncogene. 1994;9:699–705. - PubMed
-
- Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. The FEBS journal. 2006;273:5231–5244. - PubMed
-
- Lafdil F, Chobert MN, Deveaux V, Zafrani ES, Mavier P, Nakano T, Laperche Y, Brouillet A. Growth arrest-specific protein 6 deficiency impairs liver tissue repair after acute toxic hepatitis in mice. Journal of hepatology. 2009;51:55–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

