Inhibition of RAC1 GTPase sensitizes pancreatic cancer cells to γ-irradiation
- PMID: 25344910
- PMCID: PMC4279370
- DOI: 10.18632/oncotarget.2500
Inhibition of RAC1 GTPase sensitizes pancreatic cancer cells to γ-irradiation
Erratum in
-
Correction: Inhibition of RAC1 GTPase sensitizes pancreatic cancer cells to γ-irradiation.Oncotarget. 2020 Jan 21;11(3):304. doi: 10.18632/oncotarget.27450. eCollection 2020 Jan 21. Oncotarget. 2020. PMID: 32076492 Free PMC article.
Abstract
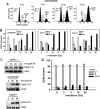
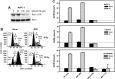
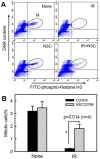
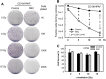
Radiation therapy is a staple treatment for pancreatic cancer. However, owing to the intrinsic radioresistance of pancreatic cancer cells, radiation therapy often fails to increase survival of pancreatic cancer patients. Radiation impedes cancer cells by inducing DNA damage, which can activate cell cycle checkpoints. Normal cells possess both a G1 and G2 checkpoint. However, cancer cells are often defective in G1 checkpoint due to mutations/alterations in key regulators of this checkpoint. Accordingly, our results show that normal pancreatic ductal cells respond to ionizing radiation (IR) with activation of both checkpoints whereas pancreatic cancer cells respond to IR with G2/M arrest only. Overexpression/hyperactivation of Rac1 GTPase is detected in the majority of pancreatic cancers. Rac1 plays important roles in survival and Ras-mediated transformation. Here, we show that Rac1 also plays a critical role in the response of pancreatic cancer cells to IR. Inhibition of Rac1 using specific inhibitor and dominant negative Rac1 mutant not only abrogates IR-induced G2 checkpoint activation, but also increases radiosensitivity of pancreatic cancer cells through induction of apoptosis. These results implicate Rac1 signaling in the survival of pancreatic cancer cells following IR, raising the possibility that this pathway contributes to the intrinsic radioresistance of pancreatic cancer.
Figures







References
-
- Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. - PubMed
-
- Aref A, Berri R. Role of Radiation Therapy in the Management of Locally Advanced Pancreatic Cancer. Journal of Clinical Oncology. 2012;30:1564–1565. - PubMed
-
- Blackstock AW, Bernard SA, Richards F, Eagle KS, Case LD, Poole ME, Savage PD, Tepper JE. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208–2212. - PubMed
-
- Brade A, Brierley J, Oza A, Gallinger S, Cummings B, Maclean M, Pond GR, Hedley D, Wong S, Townsley C, Brezden-Masley C, Moore M. Concurrent gemcitabine and radiotherapy with and without neoadjuvant gemcitabine for locally advanced unresectable or resected pancreatic cancer: a phase I-II study. Int J Radiat Oncol Biol Phys. 2007;67:1027–1036. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

