Congo red modulates ACh-induced Ca(2+) oscillations in single pancreatic acinar cells of mice
- PMID: 25345744
- PMCID: PMC4261129
- DOI: 10.1038/aps.2014.94
Congo red modulates ACh-induced Ca(2+) oscillations in single pancreatic acinar cells of mice
Abstract
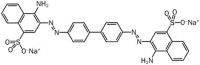
Aim: Congo red, a secondary diazo dye, is usually used as an indicator for the presence of amyloid fibrils. Recent studies show that congo red exerts neuroprotective effects in a variety of models of neurodegenerative diseases. However, its pharmacological profile remains unknown. In this study, we investigated the effects of congo red on ACh-induced Ca(2+) oscillations in mouse pancreatic acinar cells in vitro.
Methods: Acutely dissociated pancreatic acinar cells of mice were prepared. A U-tube drug application system was used to deliver drugs into the bath. Intracellular Ca(2+) oscillations were monitored by whole-cell recording of Ca(2+)-activated Cl(-) currents and by using confocal Ca(2+) imaging. For intracellular drug application, the drug was added in pipette solution and diffused into cell after the whole-cell configuration was established.
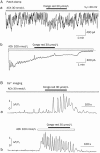
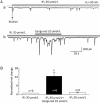
Results: Bath application of ACh (10 nmol/L) induced typical Ca(2+) oscillations in dissociated pancreatic acinar cells. Addition of congo red (1, 10, 100 μmol/L) dose-dependently enhanced Ach-induced Ca(2+) oscillations, but congo red alone did not induce any detectable response. Furthermore, this enhancement depended on the concentrations of ACh: congo red markedly enhanced the Ca(2+) oscillations induced by ACh (10-30 nmol/L), but did not alter the Ca(2+) oscillations induced by ACh (100-10000 nmol/L). Congo red also enhanced the Ca(2+) oscillations induced by bath application of IP3 (30 μmol/L). Intracellular application of congo red failed to alter ACh-induced Ca(2+) oscillations.
Conclusion: Congo red significantly modulates intracellular Ca(2+) signaling in pancreatic acinar cells, and this pharmacological effect should be fully considered when developing congo red as a novel therapeutic drug.
Figures




Similar articles
-
Cannabinoid receptor subtype 2 (CB2R) agonist, GW405833 reduces agonist-induced Ca(2+) oscillations in mouse pancreatic acinar cells.Sci Rep. 2016 Jul 19;6:29757. doi: 10.1038/srep29757. Sci Rep. 2016. PMID: 27432473 Free PMC article.
-
Duck pancreatic acinar cell as a unique model for independent cholinergic stimulation-secretion coupling.Cell Mol Neurobiol. 2009 Jul;29(5):747-56. doi: 10.1007/s10571-009-9400-8. Epub 2009 Apr 16. Cell Mol Neurobiol. 2009. PMID: 19370412 Free PMC article.
-
Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells.Gastroenterology. 2008 Aug;135(2):632-41. doi: 10.1053/j.gastro.2008.05.026. Epub 2008 May 7. Gastroenterology. 2008. PMID: 18555802
-
Berberine inhibits intracellular Ca2+ signals in mouse pancreatic acinar cells through M3 muscarinic receptors: Novel target, mechanism, and implication.Biochem Pharmacol. 2024 Jul;225:116279. doi: 10.1016/j.bcp.2024.116279. Epub 2024 May 11. Biochem Pharmacol. 2024. PMID: 38740221
-
Calcium signaling of pancreatic acinar cells in the pathogenesis of pancreatitis.World J Gastroenterol. 2014 Nov 21;20(43):16146-52. doi: 10.3748/wjg.v20.i43.16146. World J Gastroenterol. 2014. PMID: 25473167 Free PMC article. Review.
Cited by
-
The 2-aminoethoxydiphenyl borate analog, DPB161 blocks store-operated Ca2+ entry in acutely dissociated rat submandibular cells.Oncotarget. 2017 Jun 27;8(37):61551-61560. doi: 10.18632/oncotarget.18623. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977884 Free PMC article.
-
Cannabinoid receptor subtype 2 (CB2R) agonist, GW405833 reduces agonist-induced Ca(2+) oscillations in mouse pancreatic acinar cells.Sci Rep. 2016 Jul 19;6:29757. doi: 10.1038/srep29757. Sci Rep. 2016. PMID: 27432473 Free PMC article.
-
Endogenous Amyloid-formed Ca2+-permeable Channels in Aged 3xTg AD Mice.Function (Oxf). 2023 May 26;4(4):zqad025. doi: 10.1093/function/zqad025. eCollection 2023. Function (Oxf). 2023. PMID: 37342418 Free PMC article.
References
-
- Carter DB, Chou KC. A model for structure-dependent binding of Congo red to Alzheimer beta-amyloid fibrils. Neurobiol Aging. 1998;19:37–40. - PubMed
-
- Inouye H, Kirschner DA. Alzheimer's beta-amyloid: insights into fibril formation and structure from Congo red binding. Subcell Biochem. 2005;38:203–24. - PubMed
-
- Khurana R, Uversky VN, Nielsen L, Fink AL. Is Congo red an amyloid-specific dye. J Biol Chem. 2001;276:22715–21. - PubMed
-
- Lendel C, Bolognesi B, Wahlström A, Dobson CM, Gräslund A. Detergent-like interaction of Congo red with the amyloid beta peptide. Biochemistry. 2010;49:1358–60. - PubMed
-
- Wang CC, Huang HB, Tsay HJ, Shiao MS, Wu WJ, Cheng YC, et al. Characterization of Abeta aggregation mechanism probed by congo red. J Biomol Struct Dyn. 2012;30:160–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

