Naïve-like conversion enhances the difference in innate in vitro differentiation capacity between rabbit ES cells and iPS cells
- PMID: 25345855
- PMCID: PMC4354226
- DOI: 10.1262/jrd.2014-098
Naïve-like conversion enhances the difference in innate in vitro differentiation capacity between rabbit ES cells and iPS cells
Abstract
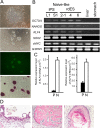
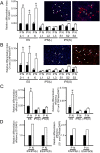
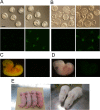
Quality evaluation of pluripotent stem cells using appropriate animal models needs to be improved for human regenerative medicine. Previously, we demonstrated that although the in vitro neural differentiating capacity of rabbit induced pluripotent stem cells (iPSCs) can be mitigated by improving their baseline level of pluripotency, i.e., by converting them into the so-called "naïve-like" state, the effect after such conversion of rabbit embryonic stem cells (ESCs) remains to be elucidated. Here we found that naïve-like conversion enhanced the differences in innate in vitro differentiation capacity between ESCs and iPSCs. Naïve-like rabbit ESCs exhibited several features indicating pluripotency, including the capacity for teratoma formation. They differentiated into mature oligodendrocytes much more effectively (3.3-7.2 times) than naïve-like iPSCs. This suggests an inherent variation in differentiation potential in vitro among PSC lines. When naïve-like ESCs were injected into preimplantation rabbit embryos, although they contributed efficiently to forming the inner cell mass of blastocysts, no chimeric pups were obtained. Thus, in vitro neural differentiation following naïve-like conversion is a promising option for determining the quality of PSCs without the need to demonstrate chimeric contribution. These results provide an opportunity to evaluate which pluripotent stem cells or treatments are best suited for therapeutic use.
Figures
References
-
- Honda A, Hirose M, Inoue K, Ogonuki N, Miki H, Shimozawa N, Hatori M, Shimizu N, Murata T, Hirose M, Katayama K, Wakisaka N, Miyoshi H, Yokoyama KK, Sankai T, Ogura A. Stable embryonic stem cell lines in rabbits: potential small animal models for human research. Reprod Biomed Online 2008; 17: 706–715. - PubMed
-
- Graur D, Duret L, Gouy M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 1996; 379: 333–335. - PubMed
-
- Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Correlation of vulnerable coronary plaques to sudden cardiac events. Lessons from a myocardial infarction-prone animal model (the WHHLMI rabbit). J Atheroscler Thromb 2004; 11: 184–189. - PubMed
-
- Weekers F, Van Herck E, Coopmans W, Michalaki M, Bowers CY, Veldhuis JD, Van den Berghe G. A novel in vivo rabbit model of hypercatabolic critical illness reveals a biphasic neuroendocrine stress response. Endocrinology 2002; 143: 764–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

