The 18 kDa translocator protein, microglia and neuroinflammation
- PMID: 25345894
- PMCID: PMC8029074
- DOI: 10.1111/bpa.12196
The 18 kDa translocator protein, microglia and neuroinflammation
Abstract
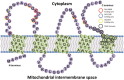
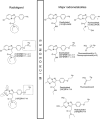
The 18 kDa translocator protein (TSPO), previously known as the peripheral benzodiazepine receptor, is expressed in the injured brain. It has become known as an imaging marker of "neuroinflammation" indicating active disease, and is best interpreted as a nondiagnostic biomarker and disease staging tool that refers to histopathology rather than disease etiology. The therapeutic potential of TSPO as a drug target is mostly based on the understanding that it is an outer mitochondrial membrane protein required for the translocation of cholesterol, which thus regulates the rate of steroid synthesis. This pivotal role together with the evolutionary conservation of TSPO has underpinned the belief that any loss or mutation of TSPO should be associated with significant physiological deficits or be outright incompatible with life. However, against prediction, full Tspo knockout mice are viable and across their lifespan do not show the phenotype expected if cholesterol transport and steroid synthesis were significantly impaired. Thus, the "translocation" function of TSPO remains to be better substantiated. Here, we discuss the literature before and after the introduction of the new nomenclature for TSPO and review some of the newer findings. In light of the controversy surrounding the function of TSPO, we emphasize the continued importance of identifying compounds with confirmed selectivity and suggest that TSPO expression is analyzed within specific disease contexts rather than merely equated with the reified concept of "neuroinflammation."
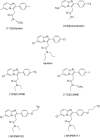
Keywords: PBR111; PET; PK11195; TSPO; microglia; neuroinflammation.
© 2014 International Society of Neuropathology.
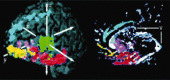
Figures






References
-
- Adam D (2002) Citation analysis: the counting house. Nature 415:726–729. - PubMed
-
- Alenfall J, Kant R, Batra S (1998) Cytotoxic effects of 125I‐labeled PBZr ligand PK 11195 in prostatic tumor cells: therapeutic implications. Cancer Lett 134:187–192. - PubMed
-
- Anholt RR, De Souza EB, Kuhar MJ, Snyder SH (1985) Depletion of peripheral‐type benzodiazepine receptors after hypophysectomy in rat adrenal gland and testis. Eur J Pharmacol 110:41–46. - PubMed
-
- Anholt RR, De Souza EB, Oster‐Granite ML, Snyder SH (1985) Peripheral‐type benzodiazepine receptors: autoradiographic localization in whole‐body sections of neonatal rats. J Pharmacol Exp Ther 233:517–526. - PubMed
-
- Anholt RR, Pedersen PL, De Souza EB, Snyder SH (1986) The peripheral‐type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem 261:576–583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

