An overview of pre-ribosomal RNA processing in eukaryotes
- PMID: 25346433
- PMCID: PMC4361047
- DOI: 10.1002/wrna.1269
An overview of pre-ribosomal RNA processing in eukaryotes
Abstract
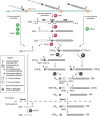
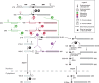
Ribosomal RNAs are the most abundant and universal noncoding RNAs in living organisms. In eukaryotes, three of the four ribosomal RNAs forming the 40S and 60S subunits are borne by a long polycistronic pre-ribosomal RNA. A complex sequence of processing steps is required to gradually release the mature RNAs from this precursor, concomitant with the assembly of the 79 ribosomal proteins. A large set of trans-acting factors chaperone this process, including small nucleolar ribonucleoparticles. While yeast has been the gold standard for studying the molecular basis of this process, recent technical advances have allowed to further define the mechanisms of ribosome biogenesis in animals and plants. This renewed interest for a long-lasting question has been fueled by the association of several genetic diseases with mutations in genes encoding both ribosomal proteins and ribosome biogenesis factors, and by the perspective of new anticancer treatments targeting the mechanisms of ribosome synthesis. A consensus scheme of pre-ribosomal RNA maturation is emerging from studies in various kinds of eukaryotic organisms. However, major differences between mammalian and yeast pre-ribosomal RNA processing have recently come to light.
© 2014 The Authors. WIREs RNA published by John Wiley & Sons, Ltd.
Figures


References
-
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Ban N. Atomic structures of the eukaryotic ribosome. Trends Biochem Sci. 2012;37:189–198. - PubMed
-
- Yusupova G, Yusupov M. High-resolution structure of the eukaryotic 80S ribosome. Annu Rev Biochem. 2014;83:467–486. - PubMed
-
- Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci. 1999;24:437–440. - PubMed
-
- Hernandez-Verdun D, Roussel P, Thiry M, Sirri V, Lafontaine DL. The nucleolus: structure/function relationship in RNA metabolism. WIREs RNA. 2010;1:415–431. - PubMed
-
- Babu KA, Verma RS. Structural and functional aspects of nucleolar organizer regions (NORs) of human chromosomes. Int Rev Cytol. 1985;94:151–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

