Motor neurons and the generation of spinal motor neuron diversity
- PMID: 25346659
- PMCID: PMC4191298
- DOI: 10.3389/fncel.2014.00293
Motor neurons and the generation of spinal motor neuron diversity
Abstract
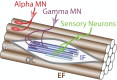
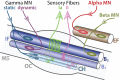
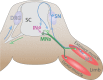
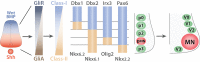
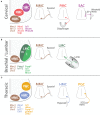
Motor neurons (MNs) are neuronal cells located in the central nervous system (CNS) controlling a variety of downstream targets. This function infers the existence of MN subtypes matching the identity of the targets they innervate. To illustrate the mechanism involved in the generation of cellular diversity and the acquisition of specific identity, this review will focus on spinal MNs (SpMNs) that have been the core of significant work and discoveries during the last decades. SpMNs are responsible for the contraction of effector muscles in the periphery. Humans possess more than 500 different skeletal muscles capable to work in a precise time and space coordination to generate complex movements such as walking or grasping. To ensure such refined coordination, SpMNs must retain the identity of the muscle they innervate. Within the last two decades, scientists around the world have produced considerable efforts to elucidate several critical steps of SpMNs differentiation. During development, SpMNs emerge from dividing progenitor cells located in the medial portion of the ventral neural tube. MN identities are established by patterning cues working in cooperation with intrinsic sets of transcription factors. As the embryo develop, MNs further differentiate in a stepwise manner to form compact anatomical groups termed pools connecting to a unique muscle target. MN pools are not homogeneous and comprise subtypes according to the muscle fibers they innervate. This article aims to provide a global view of MN classification as well as an up-to-date review of the molecular mechanisms involved in the generation of SpMN diversity. Remaining conundrums will be discussed since a complete understanding of those mechanisms constitutes the foundation required for the elaboration of prospective MN regeneration therapies.
Keywords: central nervous system; development; lower motor neuron; motor neurons; spinal cord; spinal motor neuron; transcription factors.
Figures




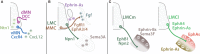
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

