Changes in the response properties of inferior colliculus neurons relating to tinnitus
- PMID: 25346722
- PMCID: PMC4191193
- DOI: 10.3389/fneur.2014.00203
Changes in the response properties of inferior colliculus neurons relating to tinnitus
Abstract
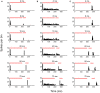
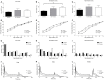
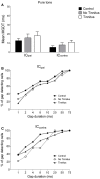
Tinnitus is often identified in animal models by using the gap prepulse inhibition of acoustic startle. Impaired gap detection following acoustic over-exposure (AOE) is thought to be caused by tinnitus "filling in" the gap, thus, reducing its salience. This presumably involves altered perception, and could conceivably be caused by changes at the level of the neocortex, i.e., cortical reorganization. Alternatively, reduced gap detection ability might reflect poorer temporal processing in the brainstem, caused by AOE; in which case, impaired gap detection would not be a reliable indicator of tinnitus. We tested the latter hypothesis by examining gap detection in inferior colliculus (IC) neurons following AOE. Seven of nine unilaterally noise-exposed guinea pigs exhibited behavioral evidence of tinnitus. In these tinnitus animals, neural gap detection thresholds (GDTs) in the IC significantly increased in response to broadband noise stimuli, but not to pure tones or narrow-band noise. In addition, when IC neurons were sub-divided according to temporal response profile (onset vs. sustained firing patterns), we found a significant increase in the proportion of onset-type responses after AOE. Importantly, however, GDTs were still considerably shorter than gap durations commonly used in objective behavioral tests for tinnitus. These data indicate that the neural changes observed in the IC are insufficient to explain deficits in behavioral gap detection that are commonly attributed to tinnitus. The subtle changes in IC neuron response profiles following AOE warrant further investigation.
Keywords: acoustic over-exposure; auditory; behavior; electrophysiology; gap detection; response types; tinnitus; tinnitus animal model.
Figures




Similar articles
-
Variable Effects of Acoustic Trauma on Behavioral and Neural Correlates of Tinnitus In Individual Animals.Front Behav Neurosci. 2016 Oct 25;10:207. doi: 10.3389/fnbeh.2016.00207. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27826232 Free PMC article.
-
Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs.Front Syst Neurosci. 2012 May 31;6:42. doi: 10.3389/fnsys.2012.00042. eCollection 2012. Front Syst Neurosci. 2012. PMID: 22666193 Free PMC article.
-
Transcriptional profile changes caused by noise-induced tinnitus in the cochlear nucleus and inferior colliculus of the rat.Ann Med. 2024 Dec;56(1):2402949. doi: 10.1080/07853890.2024.2402949. Epub 2024 Sep 13. Ann Med. 2024. PMID: 39268590 Free PMC article.
-
Tinnitus-related changes in the inferior colliculus.Front Neurol. 2015 Mar 30;6:61. doi: 10.3389/fneur.2015.00061. eCollection 2015. Front Neurol. 2015. PMID: 25870582 Free PMC article. Review.
-
Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity.Hear Res. 2011 Sep;279(1-2):111-7. doi: 10.1016/j.heares.2011.04.004. Epub 2011 Apr 21. Hear Res. 2011. PMID: 21527325 Free PMC article. Review.
Cited by
-
Effect of Unilateral Acoustic Trauma on Neuronal Firing Activity in the Inferior Colliculus of Mice.Front Synaptic Neurosci. 2021 Jun 22;13:684141. doi: 10.3389/fnsyn.2021.684141. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34239435 Free PMC article.
-
Effects of the cannabinoid CB1 agonist ACEA on salicylate ototoxicity, hyperacusis and tinnitus in guinea pigs.Hear Res. 2017 Dec;356:51-62. doi: 10.1016/j.heares.2017.10.012. Epub 2017 Oct 31. Hear Res. 2017. PMID: 29108871 Free PMC article.
-
Embryonic medial ganglionic eminence cells survive and integrate into the inferior colliculus of adult mice.Hear Res. 2022 Jul;420:108520. doi: 10.1016/j.heares.2022.108520. Epub 2022 May 16. Hear Res. 2022. PMID: 35617926 Free PMC article.
-
The Pathological Mechanisms and Treatments of Tinnitus.Discoveries (Craiova). 2021 Sep 30;9(3):e137. doi: 10.15190/d.2021.16. eCollection 2021 Jul-Sep. Discoveries (Craiova). 2021. PMID: 35350720 Free PMC article. Review.
-
What's the buzz? The neuroscience and the treatment of tinnitus.Physiol Rev. 2021 Oct 1;101(4):1609-1632. doi: 10.1152/physrev.00029.2020. Epub 2021 Mar 26. Physiol Rev. 2021. PMID: 33769102 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

