FaPOD27 functions in the metabolism of polyphenols in strawberry fruit (Fragaria sp.)
- PMID: 25346738
- PMCID: PMC4191155
- DOI: 10.3389/fpls.2014.00518
FaPOD27 functions in the metabolism of polyphenols in strawberry fruit (Fragaria sp.)
Abstract
The strawberry (Fragaria × ananassa) is one of the most preferred fresh fruit worldwide, accumulates numerous flavonoids but has limited shelf life due to excessive tissue softening caused by cell wall degradation. Since lignin is one of the polymers that strengthen plant cell walls and might contribute to some extent to fruit firmness monolignol biosynthesis was studied in strawberry fruit. Cinnamoyl-CoA reductase (CCR), cinnamyl alcohol dehydrogenase (CAD), and a peroxidase (POD27) gene were strongly expressed in red, ripe fruit whereas a second POD gene was primarily expressed in green, immature fruit. Moreover, FaPOD27 transcripts were strongly and constitutively induced in fruits exposed to Agrobacterium infection. Gene expression levels and enzymatic activities of FaCCR and FaCAD were efficiently suppressed through RNAi in FaCCR- and FaCAD-silenced strawberries. Besides, significantly elevated FaPOD transcript levels were detected after agroinfiltration of pBI-FaPOD constructs in fruits. At the same time, levels of G-monomers were considerably reduced in FaCCR-silenced fruits whereas the proportion of both G- and S-monomers decisively decreased in FaCAD-silenced and pBI-FaPOD fruits. Development, firmness, and lignin level of the treated fruits were similar to pBI-intron control fruits, presumably attributed to increased expression levels of FaPOD27 upon agroinfiltration. Additionally, enhanced firmness, accompanied with elevated lignin levels, was revealed in chalcone synthase-deficient fruits (CHS(-)), independent of down- or up-regulation of individual and combined FaCCR. FaCAD, and FaPOD genes by agroinfiltration, when compared to CHS(-)/pBI-intron control fruits. These approaches provide further insight into the genetic control of flavonoid and lignin synthesis in strawberries. The results suggest that FaPOD27 is a key gene for lignin biosynthesis in strawberry fruit and thus to improving the firmness of strawberries.
Keywords: fruit firmness; lignification; monolignol genes; peroxidase; strawberry.
Figures

 ) or down-regulated (
) or down-regulated ( ) when fruits were agroinfiltrated pBI-intron (control constructs), down- or up-regulation of individual genes (FaCCR. FaCAD, or FaPOD) (Fig. S12). The green shade indicating p-coumaroyl-CoA is the common substrate of CHS, HCT, and CCR. Red CCR. CAD, and POD indicate down- and up-regulated genes.
) when fruits were agroinfiltrated pBI-intron (control constructs), down- or up-regulation of individual genes (FaCCR. FaCAD, or FaPOD) (Fig. S12). The green shade indicating p-coumaroyl-CoA is the common substrate of CHS, HCT, and CCR. Red CCR. CAD, and POD indicate down- and up-regulated genes.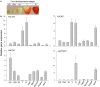







Similar articles
-
Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit.Plant Physiol Biochem. 2013 Sep;70:433-44. doi: 10.1016/j.plaphy.2013.06.008. Epub 2013 Jun 21. Plant Physiol Biochem. 2013. PMID: 23835361
-
RNAi-induced silencing of gene expression in strawberry fruit (Fragaria x ananassa) by agroinfiltration: a rapid assay for gene function analysis.Plant J. 2006 Dec;48(5):818-26. doi: 10.1111/j.1365-313X.2006.02913.x. Epub 2006 Nov 8. Plant J. 2006. PMID: 17092319
-
Redirection of flavonoid biosynthesis through the down-regulation of an anthocyanidin glucosyltransferase in ripening strawberry fruit.Plant Physiol. 2008 Apr;146(4):1528-39. doi: 10.1104/pp.107.114280. Epub 2008 Feb 7. Plant Physiol. 2008. PMID: 18258692 Free PMC article.
-
Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity.Phytochemistry. 2002 Oct;61(3):221-94. doi: 10.1016/s0031-9422(02)00211-x. Phytochemistry. 2002. PMID: 12359514 Review.
-
Recent advances in portable devices for fruit firmness assessment.Crit Rev Food Sci Nutr. 2023;63(8):1143-1154. doi: 10.1080/10408398.2021.1960477. Epub 2021 Aug 5. Crit Rev Food Sci Nutr. 2023. PMID: 34351808 Review.
Cited by
-
Priming of Defense Systems and Upregulation of MYC2 and JAZ1 Genes after Botrytis cinerea Inoculation in Methyl Jasmonate-Treated Strawberry Fruits.Plants (Basel). 2020 Apr 2;9(4):447. doi: 10.3390/plants9040447. Plants (Basel). 2020. PMID: 32252456 Free PMC article.
-
The Genetics of Differential Gene Expression Related to Fruit Traits in Strawberry (Fragaria ×ananassa).Front Genet. 2020 Feb 7;10:1317. doi: 10.3389/fgene.2019.01317. eCollection 2019. Front Genet. 2020. PMID: 32117406 Free PMC article.
-
Functional characterization of COMT genes in Chinese white pear (Pyrus bretschneideri) and their role in lignin synthesis.Front Plant Sci. 2025 Jun 9;16:1614220. doi: 10.3389/fpls.2025.1614220. eCollection 2025. Front Plant Sci. 2025. PMID: 40551766 Free PMC article.
-
Activation of Shikimate, Phenylpropanoid, Oxylipins, and Auxin Pathways in Pectobacterium carotovorum Elicitors-Treated Moss.Front Plant Sci. 2016 Mar 22;7:328. doi: 10.3389/fpls.2016.00328. eCollection 2016. Front Plant Sci. 2016. PMID: 27047509 Free PMC article.
-
Transient transformation meets gene function discovery: the strawberry fruit case.Front Plant Sci. 2015 Jun 12;6:444. doi: 10.3389/fpls.2015.00444. eCollection 2015. Front Plant Sci. 2015. PMID: 26124771 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

