T-Lymphocyte Deficiency Exacerbates Behavioral Deficits in the 6-OHDA Unilateral Lesion Rat Model for Parkinson's Disease
- PMID: 25346865
- PMCID: PMC4207300
- DOI: 10.4172/2155-9562.1000209
T-Lymphocyte Deficiency Exacerbates Behavioral Deficits in the 6-OHDA Unilateral Lesion Rat Model for Parkinson's Disease
Abstract
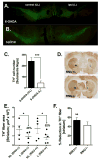
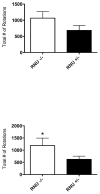
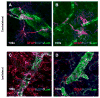
T-lymphocytes have been previously implicated in protecting dopaminergic neurons in the substantianigra from induced cell death. However, the role of T-cells in neurodegenerative models such as Parkinson's disease (PD) has not been fully elucidated. To examine the role of T-lymphocytes on motor behavior in the 6-hydroxydopamine (6-OHDA) unilateral striatal partial lesion PD rat model, we assessed progression of hemi-parkinsonian lesions in the substantia nigra, induced by 6-OHDA striatal injections, in athymic rats (RNU-/-, T-lymphocyte-deficient) as compared to RNU-/+ rats (phenotypically normal). Motor skills were determined by the cylinder and D-amphetamine sulfate-induced rotational behavioral tests. Cylinder behavioral test showed no significant difference between unilaterally lesioned RNU-/- and RNU-/+ rats. However both unilaterally lesioned RNU-/- and RNU-/+ rats favored the use of the limb ipsilateral to lesion. Additionally, amphetamine-induced rotational test revealed greater rotational asymmetry in RNU-/- rats compared to RNU-/+ rats at two- and six-week post-lesion. Quantitative immunohistochemistry confirmed loss of striatal TH-immunopositive fibers in RNU-/- and RNU-/+ rat, as well as blood-brain-barrier changes associated with PD that may influence passage of immune cells into the central nervous system in RNU-/- brains. Specifically, GFAP immunopositive cells were decreased, as were astrocytic end-feet (AQP4) contacting blood vessels (laminin) in the lesioned relative to contralateral striatum. Flow cytometric analysis in 6-OHDA lesioned RNU-/+rats revealed increased CD4+ and decreased CD8+ T cells specifically within lesioned brain. These results suggest that both major T cell subpopulations are significantly and reciprocally altered following 6-OHDA-lesioning, and that global T cell deficiency exacerbates motor behavioral defects in this rat model of PD.
Figures
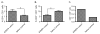
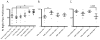

References
-
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007;82:1083–1094. - PubMed
-
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. - PubMed
-
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, et al. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–16. - PubMed
-
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11:493–498. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
