CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection
- PMID: 25347065
- PMCID: PMC4241536
- DOI: 10.7554/eLife.03180
CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection
Abstract
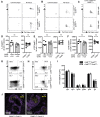
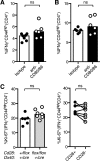
The co-stimulatory molecule CD28 is essential for activation of helper T cells. Despite this critical role, it is not known whether CD28 has functions in maintaining T cell responses following activation. To determine the role for CD28 after T cell priming, we generated a strain of mice where CD28 is removed from CD4(+) T cells after priming. We show that continued CD28 expression is important for effector CD4(+) T cells following infection; maintained CD28 is required for the expansion of T helper type 1 cells, and for the differentiation and maintenance of T follicular helper cells during viral infection. Persistent CD28 is also required for clearance of the bacterium Citrobacter rodentium from the gastrointestinal tract. Together, this study demonstrates that CD28 persistence is required for helper T cell polarization in response to infection, describing a novel function for CD28 that is distinct from its role in T cell priming.
Keywords: CD28; helper T cells; immunology; infection; mouse.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures










References
-
- Attridge K, Kenefeck R, Wardzinski L, Qureshi OS, Wang CJ, Manzotti C, Okkenhaug K, Walker LS. 2014. IL-21 promotes CD4 T Cell responses by phosphatidylinositol 3-kinase-dependent upregulation of CD86 on B cells. Journal of Immunology 192:2195–2201. doi: 10.4049/jimmunol.1302082. - DOI - PMC - PubMed
-
- Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. 2006. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. The Journal of Biological Chemistry 281:11992–12000. doi: 10.1074/jbc.M513613200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

