Methods for detecting circulating cancer stem cells (CCSCs) as a novel approach for diagnosis of colon cancer relapse/metastasis
- PMID: 25347154
- PMCID: PMC4281282
- DOI: 10.1038/labinvest.2014.133
Methods for detecting circulating cancer stem cells (CCSCs) as a novel approach for diagnosis of colon cancer relapse/metastasis
Abstract
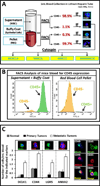
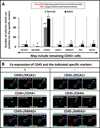
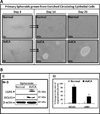
Cancer stem cells (CSCs) are believed to be resistant to currently available therapies and may be responsible for relapse of cancer in patients. Measuring circulating tumor cells (CTCs) in the blood of patients has emerged as a non-invasive diagnostic procedure for screening patients who may be at high risk for developing metastatic cancers or relapse of the cancer disease. However, accurate detection of CTCs has remained a problem, as epithelial-cell markers used to date are not always reliable for detecting CTCs, especially during epithelial-mesenchymal transition. As CSCs are required to initiate metastatic tumors, our goal was to optimize and standardize a method for identifying circulating CSCs (CCSCs) in patients, using established CSC markers. Here, we report for the first time the detection of CCSCs in the blood of athymic nude mice, bearing metastatic tumors, and in the blood of patients positive for colonic adenocarcinomas. Using a simple and non-expensive method, we isolated a relatively pure population of CSCs (CD45-/CK19+), free of red blood cells and largely free of contaminating CD45+ white blood cells. Enriched CCSCs from patients with colon adenocarcinomas had a malignant phenotype and co-expressed CSC markers (DCLK1/LGR5) with CD44/Annexin A2. CSCs were not found in the blood of non-cancer patients, free of colonic growths. Enriched CCSCs from colon cancer patients grew primary spheroids, suggesting the presence of tumor-initiating cells in the blood of these patients. In conclusion, we have developed a novel diagnostic assay for detecting CSCs in circulation, which may more accurately predict the risk of relapse or metastatic disease in patients. As CSCs can potentially initiate metastatic growths, patients positive for CCSCs can be treated with inhibitory agents that selectively target CSCs, besides conventional treatments, to reduce the risk of relapse/metastatic disease for improving clinical outcomes.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

