Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors
- PMID: 25347353
- PMCID: PMC4241193
- DOI: 10.1038/nn.3871
Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors
Abstract
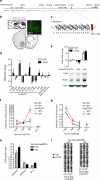
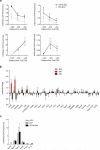
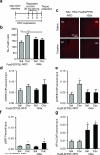
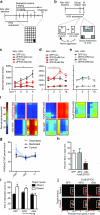
Chronic exposure to drugs of abuse or stress regulates transcription factors, chromatin-modifying enzymes and histone post-translational modifications in discrete brain regions. Given the promiscuity of the enzymes involved, it has not yet been possible to obtain direct causal evidence to implicate the regulation of transcription and consequent behavioral plasticity by chromatin remodeling that occurs at a single gene. We investigated the mechanism linking chromatin dynamics to neurobiological phenomena by applying engineered transcription factors to selectively modify chromatin at a specific mouse gene in vivo. We found that histone methylation or acetylation at the Fosb locus in nucleus accumbens, a brain reward region, was sufficient to control drug- and stress-evoked transcriptional and behavioral responses via interactions with the endogenous transcriptional machinery. This approach allowed us to relate the epigenetic landscape at a given gene directly to regulation of its expression and to its subsequent effects on reward behavior.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

