Endocannabinoids, related compounds and their metabolic routes
- PMID: 25347455
- PMCID: PMC6271436
- DOI: 10.3390/molecules191117078
Endocannabinoids, related compounds and their metabolic routes
Abstract
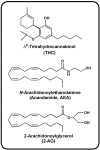
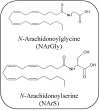
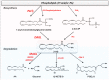
Endocannabinoids are lipid mediators able to bind to and activate cannabinoid receptors, the primary molecular targets responsible for the pharmacological effects of the Δ9-tetrahydrocannabinol. These bioactive lipids belong mainly to two classes of compounds: N-acylethanolamines and acylesters, being N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), respectively, their main representatives. During the last twenty years, an ever growing number of fatty acid derivatives (endocannabinoids and endocannabinoid-like compounds) have been discovered and their activities biological is the subject of intense investigations. Here, the most recent advances, from a therapeutic point of view, on endocannabinoids, related compounds, and their metabolic routes will be reviewed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G., Mackie K., et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

