Nerve growth factor reduces amiloride-sensitive Na+ transport in human airway epithelial cells
- PMID: 25347857
- PMCID: PMC4187554
- DOI: 10.14814/phy2.12073
Nerve growth factor reduces amiloride-sensitive Na+ transport in human airway epithelial cells
Abstract
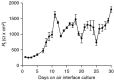
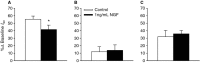
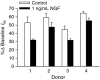
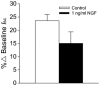
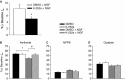
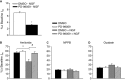
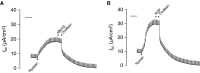
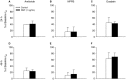
Nerve growth factor (NGF) is overexpressed in patients with inflammatory lung diseases, including virus infections. Airway surface liquid (ASL), which is regulated by epithelial cell ion transport, is essential for normal lung function. No information is available regarding the effect of NGF on ion transport of airway epithelium. To investigate whether NGF can affect ion transport, human primary air-interface cultured epithelial cells were placed in Ussing chambers to obtain transepithelial voltage (-7.1 ± 3.4 mV), short-circuit current (Isc, 5.9 ± 1.0 μA), and transepithelial resistance (750 Ω·cm(2)), and to measure responses to ion transport inhibitors. Amiloride (apical, 3.5 × 10(-5) mol/L) decreased Isc by 55.3%. Apically applied NGF (1 ng/mL) reduced Isc by 5.3% in 5 min; basolaterally applied NGF had no effect. The response to amiloride was reduced (41.6%) in the presence of NGF. K-252a (10 nmol/L, apical) did not itself affect Na(+) transport, but it attenuated the NGF-induced reduction in Na(+) transport, indicating the participation of the trkA receptor in the NGF-induced reduction in Na(+) transport. PD-98059 (30 μmol/L, apical and basolateral) did not itself affect Na(+) transport, but attenuated the NGF-induced reduction in Na(+) transport, indicating that trkA activated the Erk 1/2 signaling cascade. NGF stimulated phosphorylation of Erk 1/2 and the β-subunit of ENaC. K-252a and PD-98059 inhibited these responses. NGF had no effect on Isc in the presence of apical nystatin (50 μmol/L). These results indicate that NGF inhibits Na(+) transport through a trkA-Erk 1/2-activated signaling pathway linked to ENaC phosphorylation.
Keywords: Airway epithelium; electrophysiology; ion transport; lung; nerve growth factor.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures



References
-
- Bhalla V., Hallows K. R. 2008. Mechanisms of ENaC regulation and clinical implications. J. Am. Soc. Nephrol.; 19:1845-1854. - PubMed
-
- Braun A., Appel E., Baruch R., Herz U., Botchkarev V., Paus R. 1998. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol.; 28:3240-3251. - PubMed
-
- Chen X. J., Seth S., Yue G., Kamat P., Compans R. W., Guidot D. 2004. Influenza virus inhibits ENaC and lung fluid clearance. Am. J. Physiol. Lung Cell. Mol. Physiol.; 287:L366-L373. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

