A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study
- PMID: 25347938
- PMCID: PMC5024052
- DOI: 10.1111/dom.12403
A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study
Abstract
Aims: To assess the effects of renal impairment (RI) on the efficacy and safety of ipragliflozin in patients with type 2 diabetes mellitus (T2DM).
Methods: A cohort of Japanese patients with T2DM and mild to moderate RI and poor glycaemic control, despite diet/exercise therapy alone or diet/exercise therapy in combination with an oral hypoglycaemic agent (an α-glucosidase inhibitor, a sulfonylurea, or pioglitazone), were randomized in a double-blind manner to 50 mg ipragliflozin or placebo once daily for 24 weeks. The patients continued open-label ipragliflozin for a 28-week extension period (total treatment duration: 52 weeks).
Results: Ipragliflozin significantly decreased glycated haemoglobin (HbA1c) and fasting plasma glucose (FPG) levels and body weight from baseline to week 24 (last observation carried forward) compared with placebo in all patients with RI. The decreases in HbA1c and FPG levels were statistically significant in patients with mild RI, but not in patients with moderate RI. Ipragliflozin significantly reduced body weight in both RI groups. The improvements in glycaemic control were maintained in the 28-week extension period. Ipragliflozin was associated with no clinically significant safety concerns, and its safety profiles were not influenced by the severity of RI.
Conclusions: Ipragliflozin significantly improved glycaemic control and body weight in patients with T2DM with mild RI, but did not improve glycaemic control in patients with moderate RI. Ipragliflozin is a valid treatment option for patients with mild RI but not those with moderate RI.
Keywords: SGLT2 inhibitor.
© 2014 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
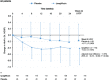


References
-
- Wright EM. Renal Na(+)‐glucose cotransporters. Am J Physiol Renal Physiol 2001; 280: F10–F18. - PubMed
-
- Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 2007; 261: 32–43. - PubMed
-
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium‐glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75: 1272–1277. - PubMed
-
- Krook A, Kawano Y, Song XM et al. Improved glucose tolerance restores insulin‐stimulated Akt kinase activity and glucose transport in skeletal muscle from diabetic Goto‐Kakizaki rats. Diabetes 1997; 46: 2110–2114. - PubMed
-
- Tahara A, Kurosaki E, Yokono M et al. Antidiabetic effects of SGLT2‐selective inhibitor ipragliflozin in streptozotocin‐nicotinamide‐induced mildly diabetic mice. J Pharmacol Sci 2012; 120: 36–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

