Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children's Oncology Group Study AALL07P4
- PMID: 25348002
- PMCID: PMC4239306
- DOI: 10.1200/JCO.2014.55.5763
Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children's Oncology Group Study AALL07P4
Abstract
Purpose: Asparaginase is a critical agent used to treat acute lymphoblastic leukemia (ALL). Pegaspargase (SS-PEG), a pegylated form of Escherichia coli L-asparaginase with a succinimidyl succinate (SS) linker, is the first-line asparaginase product used in Children's Oncology Group (COG) ALL trials. Calaspargase pegol (SC-PEG) replaces the SS linker in SS-PEG with a succinimidyl carbamate linker, creating a more stable molecule. COG AALL07P4 was designed to determine the pharmacokinetic and pharmacodynamic comparability of SC-PEG to SS-PEG in patients with newly diagnosed high-risk (HR) B-cell ALL.
Patients and methods: A total of 165 evaluable patients were randomly assigned at a 2:1 ratio to receive SC-PEG at 2,100 (SC-PEG2100; n = 69) or 2,500 IU/m(2) (SC-PEG2500; n = 42) versus SS-PEG 2,500 IU/m(2) (SS-PEG2500; n = 54) as part of an otherwise identical chemotherapy regimen. The groups were similar demographically, except more female patients received SC-PEG2500.
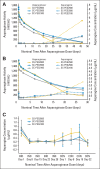
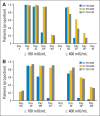
Results: The mean half-life of plasma asparaginase activity for both SC-PEG doses was approximately 2.5× longer than that of SS-PEG2500. The total systemic exposure, as defined by induction area under the curve from time 0 to 25 days, was greater with SC-PEG2500 than with SS-PEG2500 or SC-PEG2100. The proportion of patients with plasma asparaginase activity ≥ 100 mIU/mL and ≥ 400 mIU/mL was higher in patients who received SC-PEG as compared with SS-PEG2500. After one dose of pegylated asparaginase on induction day 4, plasma asparagine was undetectable for 11 days for SS-PEG2500 and 18 days for both SC-PEG groups.
Conclusion: SC-PEG2500 achieves a significantly longer period of asparaginase activity above defined thresholds and asparagine depletion compared with SS-PEG2500 and has a comparable toxicity profile in children with HR B-cell ALL.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Pegaspargase: A Review in Acute Lymphoblastic Leukaemia.Drugs. 2019 May;79(7):767-777. doi: 10.1007/s40265-019-01120-1. Drugs. 2019. PMID: 31030380 Free PMC article. Review.
-
Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial.Lancet Oncol. 2015 Dec;16(16):1677-90. doi: 10.1016/S1470-2045(15)00363-0. Epub 2015 Nov 6. Lancet Oncol. 2015. PMID: 26549586 Clinical Trial.
-
Efficacy and Toxicity of Pegaspargase and Calaspargase Pegol in Childhood Acute Lymphoblastic Leukemia: Results of DFCI 11-001.J Clin Oncol. 2021 Nov 1;39(31):3496-3505. doi: 10.1200/JCO.20.03692. Epub 2021 Jul 6. J Clin Oncol. 2021. PMID: 34228505 Clinical Trial.
-
Polyethylene Glycol-conjugated L-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children's Oncology Group Study (POG 8866).J Pediatr Hematol Oncol. 2011 Dec;33(8):610-6. doi: 10.1097/MPH.0b013e31822d4d4e. J Pediatr Hematol Oncol. 2011. PMID: 22042277 Free PMC article. Clinical Trial.
-
Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future.Clin Pharmacokinet. 2005;44(4):367-93. doi: 10.2165/00003088-200544040-00003. Clin Pharmacokinet. 2005. PMID: 15828851 Review.
Cited by
-
Recombinant Erwinia asparaginase (JZP458) in acute lymphoblastic leukemia: results from the phase 2/3 AALL1931 study.Blood. 2023 Feb 16;141(7):704-712. doi: 10.1182/blood.2022016923. Blood. 2023. PMID: 36108304 Free PMC article. Clinical Trial.
-
Pegaspargase: A Review in Acute Lymphoblastic Leukaemia.Drugs. 2019 May;79(7):767-777. doi: 10.1007/s40265-019-01120-1. Drugs. 2019. PMID: 31030380 Free PMC article. Review.
-
PEG-asparaginase treatment regimens for acute lymphoblastic leukaemia in children: a network meta-analysis.Cochrane Database Syst Rev. 2023 May 31;5(5):CD014570. doi: 10.1002/14651858.CD014570.pub2. Cochrane Database Syst Rev. 2023. PMID: 37260073 Free PMC article.
-
Clinical Utility of Pegaspargase in Children, Adolescents and Young Adult Patients with Acute Lymphoblastic Leukemia: A Review.Blood Lymphat Cancer. 2021 Apr 19;11:25-40. doi: 10.2147/BLCTT.S245210. eCollection 2021. Blood Lymphat Cancer. 2021. PMID: 33907490 Free PMC article. Review.
-
Plasma asparaginase activity and asparagine depletion in acute lymphoblastic leukemia patients treated with pegaspargase on Children's Oncology Group AALL07P4.Leuk Lymphoma. 2019 Jul;60(7):1740-1748. doi: 10.1080/10428194.2018.1542146. Epub 2019 Jan 10. Leuk Lymphoma. 2019. PMID: 30626253 Free PMC article. Clinical Trial.
References
-
- Gaithersburg, MD: Sigma Tau Pharmaceuticals; 2007. Investigator brochure for EZN-2285 (SC-PEG E coli L-asparaginase), version 1.
-
- US Department of Health and Human Services. Guidance for Industry: Statistical Approaches to Establishing Bioequivalence. http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf.
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

