Primary age-related tauopathy (PART): a common pathology associated with human aging
- PMID: 25348064
- PMCID: PMC4257842
- DOI: 10.1007/s00401-014-1349-0
Primary age-related tauopathy (PART): a common pathology associated with human aging
Abstract
We recommend a new term, "primary age-related tauopathy" (PART), to describe a pathology that is commonly observed in the brains of aged individuals. Many autopsy studies have reported brains with neurofibrillary tangles (NFTs) that are indistinguishable from those of Alzheimer's disease (AD), in the absence of amyloid (Aβ) plaques. For these "NFT+/Aβ-" brains, for which formal criteria for AD neuropathologic changes are not met, the NFTs are mostly restricted to structures in the medial temporal lobe, basal forebrain, brainstem, and olfactory areas (bulb and cortex). Symptoms in persons with PART usually range from normal to amnestic cognitive changes, with only a minority exhibiting profound impairment. Because cognitive impairment is often mild, existing clinicopathologic designations, such as "tangle-only dementia" and "tangle-predominant senile dementia", are imprecise and not appropriate for most subjects. PART is almost universally detectable at autopsy among elderly individuals, yet this pathological process cannot be specifically identified pre-mortem at the present time. Improved biomarkers and tau imaging may enable diagnosis of PART in clinical settings in the future. Indeed, recent studies have identified a common biomarker profile consisting of temporal lobe atrophy and tauopathy without evidence of Aβ accumulation. For both researchers and clinicians, a revised nomenclature will raise awareness of this extremely common pathologic change while providing a conceptual foundation for future studies. Prior reports that have elucidated features of the pathologic entity we refer to as PART are discussed, and working neuropathological diagnostic criteria are proposed.
Figures
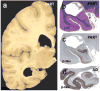
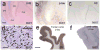

Comment in
-
Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process?Acta Neuropathol. 2014 Dec;128(6):767-72. doi: 10.1007/s00401-014-1356-1. Epub 2014 Oct 31. Acta Neuropathol. 2014. PMID: 25359108 No abstract available.
-
PART and SNAP.Acta Neuropathol. 2014 Dec;128(6):773-6. doi: 10.1007/s00401-014-1362-3. Epub 2014 Nov 8. Acta Neuropathol. 2014. PMID: 25380757 Free PMC article. No abstract available.
References
-
- Arai T, Ikeda K, Akiyama H, et al. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick's disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 2001;101:167–173. doi. - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cerebral Cortex. 1991;1:103–116. doi. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 AG013854/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- P01AG07232/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- P01 AG007232/AG/NIA NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- R173/1110/DMT_/The Dunhill Medical Trust/United Kingdom
- R01 AG041851/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P50AG08702/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- AG005131/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- G0400074/MRC_/Medical Research Council/United Kingdom
- P50 AG005136/AG/NIA NIH HHS/United States
- R01 AG011378/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- G0900652/MRC_/Medical Research Council/United Kingdom
- P01 AG019724/AG/NIA NIH HHS/United States
- G0502157/MRC_/Medical Research Council/United Kingdom
- P50 AG025688/AG/NIA NIH HHS/United States
- R01 AG038651/AG/NIA NIH HHS/United States
- AG008051/AG/NIA NIH HHS/United States
- AG184440/AG/NIA NIH HHS/United States
- P30 AG036453/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P01 AG002219/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- R01 AG037212/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

