Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets
- PMID: 25348066
- PMCID: PMC4451472
- DOI: 10.1007/s12015-014-9558-4
Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets
Abstract
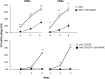
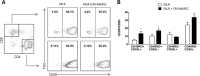
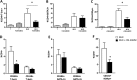
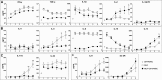
We previously demonstrated that cells derived from the mesenchymal layer of the human amniotic membrane (hAMSC) and their conditioned medium (CM-hAMSC) modulate lymphocyte proliferation in a dose-dependent manner. In order to understand the mechanisms involved in immune regulation exerted by hAMSC, we analyzed the effects of CM-hAMSC on T-cell polarization towards Th1, Th2, Th17, and T-regulatory (Treg) subsets. We show that CM-hAMSC equally suppresses the proliferation of both CD4(+) T-helper (Th) and CD8(+) cytotoxic T-lymphocytes. Moreover, we prove that the CM-hAMSC inhibitory ability affects both central (CD45RO(+)CD62L(+)) and effector memory (CD45RO(+)CD62L(-)) subsets. We evaluated the phenotype of CD4(+) cells in the MLR setting and showed that CM-hAMSC significantly reduced the expression of markers associated to the Th1 (T-bet(+)CD119(+)) and Th17 (RORγt(+)CD161(+)) populations, while having no effect on the Th2 population (GATA3(+)CD193(+)/GATA3(+)CD294(+)cells). T-cell subset modulation was substantiated through the analysis of cytokine release for 6 days during co-culture with alloreactive T-cells, whereby we observed a decrease in specific subset-related cytokines, such as a decrease in pro-inflammatory, Th1-related (TNFα, IFNγ, IL-1β), Th2 (IL-5, IL-6), Th9 (IL-9), and Th17 (IL-17A, IL-22). Furthermore, CM-hAMSC significantly induced the Treg compartment, as shown by an induction of proliferating CD4(+)FoxP3(+) cells, and an increase of CD25(+)FoxP3(+) and CD39(+)FoxP3(+) Treg in the CD4(+) population. Induction of Treg cells was corroborated by the increased secretion of TGF-β. Taken together, these data strengthen the findings regarding the immunomodulatory properties of CM-hAMSC derived from human amniotic membrane MSC, and in particular provide insights into their effect on regulation of T cell polarization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells.Stem Cell Res Ther. 2013 Jun 4;4(3):65. doi: 10.1186/scrt216. Stem Cell Res Ther. 2013. PMID: 23734780 Free PMC article.
-
Amniotic mesenchymal cells from pre-eclamptic placentae maintain immunomodulatory features as healthy controls.J Cell Mol Med. 2016 Jan;20(1):157-69. doi: 10.1111/jcmm.12715. Epub 2015 Oct 30. J Cell Mol Med. 2016. PMID: 26515425 Free PMC article.
-
IL-12 inhibits the TGF-β-dependent T cell developmental programs and skews the TGF-β-induced differentiation into a Th1-like direction.Immunobiology. 2012 Jan;217(1):74-82. doi: 10.1016/j.imbio.2011.07.032. Epub 2011 Aug 5. Immunobiology. 2012. PMID: 21903294
-
Mesenchymal stem cell effects on T-cell effector pathways.Stem Cell Res Ther. 2011 Aug 11;2(4):34. doi: 10.1186/scrt75. Stem Cell Res Ther. 2011. PMID: 21861858 Free PMC article. Review.
-
T helper subsets & regulatory T cells: rethinking the paradigm in the clinical context of solid organ transplantation.Int J Immunogenet. 2014 Jun;41(3):185-94. doi: 10.1111/iji.12106. Epub 2014 Feb 3. Int J Immunogenet. 2014. PMID: 24495112 Review.
Cited by
-
miRNAs as the important regulators of myasthenia gravis: involvement of major cytokines and immune cells.Immunol Res. 2023 Apr;71(2):153-163. doi: 10.1007/s12026-022-09342-4. Epub 2022 Nov 17. Immunol Res. 2023. PMID: 36396903 Review.
-
Human umbilical cord multipotent mesenchymal stromal cells alleviate acute ischemia-reperfusion injury of spermatogenic cells via reducing inflammatory response and oxidative stress.Stem Cell Res Ther. 2020 Jul 17;11(1):294. doi: 10.1186/s13287-020-01813-5. Stem Cell Res Ther. 2020. PMID: 32680554 Free PMC article.
-
The Cells and Extracellular Matrix of Human Amniotic Membrane Hinder the Growth and Invasive Potential of Bladder Urothelial Cancer Cells.Front Bioeng Biotechnol. 2020 Nov 9;8:554530. doi: 10.3389/fbioe.2020.554530. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33240862 Free PMC article.
-
Perinatal derivatives: How to best validate their immunomodulatory functions.Front Bioeng Biotechnol. 2022 Sep 14;10:981061. doi: 10.3389/fbioe.2022.981061. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36185431 Free PMC article. Review.
-
Amniotic Membrane Preparation Crucially Affects Its Broad-Spectrum Activity Against Uropathogenic Bacteria.Front Microbiol. 2020 Mar 24;11:469. doi: 10.3389/fmicb.2020.00469. eCollection 2020. Front Microbiol. 2020. PMID: 32265889 Free PMC article.
References
-
- Cetrulo KJ, Cetrulo CL Jr, Taghizadeh RR, editors. Perinatal stem cells. Hoboken: Wiley; 2013.
-
- Anzalone R, Iacono Lo M, Loria T, Di Stefano A, Giannuzzi P, Farina F, La Rocca G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Reviews and Reports. 2011;7(2):342–363. doi: 10.1007/s12015-010-9196-4. - DOI - PubMed
-
- La Rocca G, Corrao S, Iacono Lo M, Corsello T, Farina F, Anzalone R. Novel immunomodulatory markers expressed by human WJ-MSC: an updated review in regenerative and reparative medicine. The Open Tissue Engineering and Regenerative Medicine Journal. 2012;5:50–58. doi: 10.2174/1875043501205010050. - DOI
-
- La Rocca G, Iacono Lo M, Corsello T, Corrao S, Farina F, Anzalone R. Human Wharton’s jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: new perspectives for cellular therapy. Current Stem Cell Research & Therapy. 2013;8(1):100–113. doi: 10.2174/1574888X11308010012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

