Tolterodine extended release in the treatment of male OAB/storage LUTS: a systematic review
- PMID: 25348235
- PMCID: PMC4230346
- DOI: 10.1186/1471-2490-14-84
Tolterodine extended release in the treatment of male OAB/storage LUTS: a systematic review
Abstract
Background: Overactive bladder (OAB)/ storage lower urinary tract symptoms (LUTS) have a high prevalence affecting up to 90% of men over 80 years. The role of sufficient therapies appears crucial. In the present review, we analyzed the mechanism of action of tolterodine extended-release (ER) with the aim to clarify its efficacy and safety profile, as compared to other active treatments of OAB/storage LUTS.
Methods: A wide Medline search was performed including the combination of following words: "LUTS", "BPH", "OAB", "antimuscarinic", "tolterodine", "tolterodine ER". IPSS, IPSS storage sub-score and IPSS QoL (International Prostate Symptom Score) were the validated efficacy outcomes. In addition, the numbers of urgency episodes/24 h, urgency incontinence episodes/24 h, incontinence episodes/24 h and pad use were considered. We also evaluated the most common adverse events (AEs) reported for tolterodine ER.
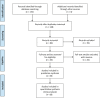
Results: Of 128 retrieved articles, 109 were excluded. The efficacy and tolerability of tolterodine ER Vs. tolterodine IR have been evaluated in a multicenter, double-blind, randomized placebo controlled study in 1529 patients with OAB. A 71% mean reduction in urgency incontinence episodes was found in the tolterodine ER group compared to a 60% reduction in the tolterodine IR (p < 0.05). Few studies evaluated the clinical efficacy of α-blocker/tolterodine combination therapy. In patients with large prostates (prostate volume >29 cc) only the combination therapy significantly reduced 24-h voiding frequency (2.8 vs. 1.7 with tamsulosin, 1.4 with tolterodine, or 1.6 with placebo). A recent meta-analysis evaluating tolterodine in comparison with other antimuscarinic drugs demonstrated that tolterodine ER was significantly more effective than placebo in reducing micturition/24 h, urinary leakage episodes/24 h, urgency episodes/24 h, and urgency incontinence episodes/24 h. With regard to adverse events, tolterodine ER was associated with a good adverse event profile resulting in the third most favorable antimuscarinic. Antimuscarinic drugs are the mainstay of pharmacological therapy for OAB / storage LUTS; several studies have demonstrated that tolterodine ER is an effective and well tolerated formulation of this class of treatment.
Conclusion: Tolterodine ER resulted effective in reducing frequency urgency and nocturia and urinary leakage in male patients with OAB/storage LUTS. Dry mouth and constipation are the most frequently reported adverse events.
Figures

References
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A, Standardisation Sub-Committee of the International Continence Society The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/S0090-4295(02)02243-4. - DOI - PubMed
-
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474–479. - PubMed
-
- Laniado ME, Ockrim JL, Marronaro A, Tubaro A, Carter SS. Serum prostate-specific antigen to predict the presence of bladder outlet obstruction in men with urinarysymptoms. BJ UInt. 2004;94:1283–1286. - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2490/14/84/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

