Targeted nanoparticles in mitochondrial medicine
- PMID: 25348382
- PMCID: PMC4397104
- DOI: 10.1002/wnan.1305
Targeted nanoparticles in mitochondrial medicine
Abstract
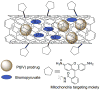
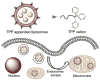
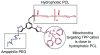
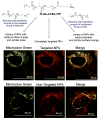
Mitochondria, the so-called 'energy factory of cells' not only produce energy but also contribute immensely in cellular mortality management. Mitochondrial dysfunctions result in various diseases including but not limited to cancer, atherosclerosis, and neurodegenerative diseases. In the recent years, targeting mitochondria emerged as an attractive strategy to control mitochondrial dysfunction-related diseases. Despite the desire to direct therapeutics to the mitochondria, the actual task is more difficult due to the highly complex nature of the mitochondria. The potential benefits of integrating nanomaterials with properties such as biodegradability, magnetization, and fluorescence into a single object of nanoscale dimensions can lead to the development of hybrid nanomedical platforms for targeting therapeutics to the mitochondria. Only a handful of nanoparticles based on metal oxides, gold nanoparticles, dendrons, carbon nanotubes, and liposomes were recently engineered to target mitochondria. Most of these materials face tremendous challenges when administered in vivo due to their limited biocompatibility. Biodegradable polymeric nanoparticles emerged as eminent candidates for effective drug delivery. In this review, we highlight the current advancements in the development of biodegradable nanoparticle platforms as effective targeting tools for mitochondrial medicine.
© 2014 Wiley Periodicals, Inc.
Figures


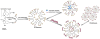
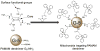
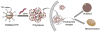
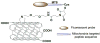


Similar articles
-
Nanoparticle-based therapeutic strategies for mitochondrial dysfunction in cardiovascular disease.J Biomed Mater Res A. 2024 Jun;112(6):895-913. doi: 10.1002/jbm.a.37668. Epub 2024 Jan 12. J Biomed Mater Res A. 2024. PMID: 38217313 Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Dual drug conjugated nanoparticle for simultaneous targeting of mitochondria and nucleus in cancer cells.ACS Appl Mater Interfaces. 2015 Apr 15;7(14):7584-98. doi: 10.1021/am5090226. Epub 2015 Apr 3. ACS Appl Mater Interfaces. 2015. PMID: 25811662
-
Delivery to mitochondria: a narrower approach for broader therapeutics.Expert Opin Drug Deliv. 2012 Aug;9(8):909-35. doi: 10.1517/17425247.2012.694864. Epub 2012 Jun 5. Expert Opin Drug Deliv. 2012. PMID: 22663303 Review.
-
Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics.Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):16288-93. doi: 10.1073/pnas.1210096109. Epub 2012 Sep 18. Proc Natl Acad Sci U S A. 2012. PMID: 22991470 Free PMC article.
Cited by
-
Recent Advances in Chemical Biology of Mitochondria Targeting.Front Chem. 2021 May 3;9:683220. doi: 10.3389/fchem.2021.683220. eCollection 2021. Front Chem. 2021. PMID: 34012953 Free PMC article. Review.
-
Novel Strategies for Disrupting Cancer-Cell Functions with Mitochondria-Targeted Antitumor Drug-Loaded Nanoformulations.Int J Nanomedicine. 2021 Jun 9;16:3907-3936. doi: 10.2147/IJN.S303832. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34135584 Free PMC article. Review.
-
Centrifugation-Free Magnetic Isolation of Functional Mitochondria Using Paramagnetic Iron Oxide Nanoparticles.Curr Protoc Cell Biol. 2017 Sep 1;76:25.4.1-25.4.20. doi: 10.1002/cpcb.26. Curr Protoc Cell Biol. 2017. PMID: 28862341 Free PMC article.
-
Photodynamic Therapy-Mediated Immune Responses in Three-Dimensional Tumor Models.Int J Mol Sci. 2021 Nov 23;22(23):12618. doi: 10.3390/ijms222312618. Int J Mol Sci. 2021. PMID: 34884424 Free PMC article. Review.
-
Uptake and Intracellular Trafficking Studies of Multiple Dye-Doped Core-Shell Silica Nanoparticles in Lymphoid and Myeloid Cells.Nanotechnol Sci Appl. 2021 Mar 8;14:29-48. doi: 10.2147/NSA.S290867. eCollection 2021. Nanotechnol Sci Appl. 2021. PMID: 33727804 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

