Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer
- PMID: 25348516
- PMCID: PMC4447091
- DOI: 10.1158/1078-0432.CCR-14-1591
Combined MEK and PI3K inhibition in a mouse model of pancreatic cancer
Abstract
Purpose: Improved therapeutic approaches are needed for the treatment of pancreatic ductal adenocarcinoma (PDAC). As dual MEK and PI3K inhibition is presently being used in clinical trials for patients with PDAC, we sought to test the efficacy of combined targeting of these pathways in PDAC using both in vitro drug screens and genetically engineered mouse models (GEMM).
Experimental design: We performed high-throughput screening of >500 human cancer cell lines (including 46 PDAC lines), for sensitivity to 50 clinically relevant compounds, including MEK and PI3K inhibitors. We tested the top hit in the screen, the MEK1/2 inhibitor, AZD6244, for efficacy alone or in combination with the PI3K inhibitors, BKM120 or GDC-0941, in a Kras(G12D)-driven GEMM that recapitulates the histopathogenesis of human PDAC.
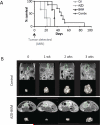
Results: In vitro screens revealed that PDAC cell lines are relatively resistant to single-agent therapies. The response profile to the MEK1/2 inhibitor, AZD6244, was an outlier, showing the highest selective efficacy in PDAC. Although MEK inhibition alone was mainly cytostatic, apoptosis was induced when combined with PI3K inhibitors (BKM120 or GDC-0941). When tested in a PDAC GEMM and compared with the single agents or vehicle controls, the combination delayed tumor formation in the setting of prevention and extended survival when used to treat advanced tumors, although no durable responses were observed.
Conclusions: Our studies point to important contributions of MEK and PI3K signaling to PDAC pathogenesis and suggest that dual targeting of these pathways may provide benefit in some patients with PDAC. Clin Cancer Res; 21(2); 396-404. ©2014 AACR.
©2014 American Association for Cancer Research.
Figures




References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. - PubMed
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

