Inhibition of endoplasmic reticulum-resident glucosidases impairs severe acute respiratory syndrome coronavirus and human coronavirus NL63 spike protein-mediated entry by altering the glycan processing of angiotensin I-converting enzyme 2
- PMID: 25348530
- PMCID: PMC4291352
- DOI: 10.1128/AAC.03999-14
Inhibition of endoplasmic reticulum-resident glucosidases impairs severe acute respiratory syndrome coronavirus and human coronavirus NL63 spike protein-mediated entry by altering the glycan processing of angiotensin I-converting enzyme 2
Abstract
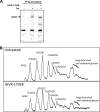
Endoplasmic reticulum (ER)-resident glucosidases I and II sequentially trim the three terminal glucose moieties on the N-linked glycans attached to nascent glycoproteins. These reactions are the first steps of N-linked glycan processing and are essential for proper folding and function of many glycoproteins. Because most of the viral envelope glycoproteins contain N-linked glycans, inhibition of ER glucosidases with derivatives of 1-deoxynojirimycin, i.e., iminosugars, efficiently disrupts the morphogenesis of a broad spectrum of enveloped viruses. However, like viral envelope proteins, the cellular receptors of many viruses are also glycoproteins. It is therefore possible that inhibition of ER glucosidases not only compromises virion production but also disrupts expression and function of viral receptors and thus inhibits virus entry into host cells. Indeed, we demonstrate here that iminosugar treatment altered the N-linked glycan structure of angiotensin I-converting enzyme 2 (ACE2), which did not affect its expression on the cell surface or its binding of the severe acute respiratory syndrome coronavirus (SARS-CoV) spike glycoprotein. However, alteration of N-linked glycans of ACE2 impaired its ability to support the transduction of SARS-CoV and human coronavirus NL63 (HCoV-NL63) spike glycoprotein-pseudotyped lentiviral particles by disruption of the viral envelope protein-triggered membrane fusion. Hence, in addition to reducing the production of infectious virions, inhibition of ER glucosidases also impairs the entry of selected viruses via a post-receptor-binding mechanism.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Role of N-linked glycosylation sites in human ACE2 in SARS-CoV-2 and hCoV-NL63 infection.J Virol. 2025 May 20;99(5):e0220224. doi: 10.1128/jvi.02202-24. Epub 2025 Mar 28. J Virol. 2025. PMID: 40152594 Free PMC article.
-
Analysis of the Role of N-Linked Glycosylation in Cell Surface Expression, Function, and Binding Properties of SARS-CoV-2 Receptor ACE2.Microbiol Spectr. 2021 Oct 31;9(2):e0119921. doi: 10.1128/Spectrum.01199-21. Epub 2021 Sep 8. Microbiol Spectr. 2021. PMID: 34494876 Free PMC article.
-
Antiviral therapies targeting host ER alpha-glucosidases: current status and future directions.Antiviral Res. 2013 Sep;99(3):251-60. doi: 10.1016/j.antiviral.2013.06.011. Epub 2013 Jun 29. Antiviral Res. 2013. PMID: 23816430 Free PMC article. Review.
-
Characterization of spike S1/S2 processing and entry pathways of lentiviral pseudoviruses bearing seasonal human coronaviruses NL63, 229E, and HKU1 spikes.Microbiol Spectr. 2025 Mar 4;13(3):e0280824. doi: 10.1128/spectrum.02808-24. Epub 2025 Jan 28. Microbiol Spectr. 2025. PMID: 39873512 Free PMC article.
-
Severe acute respiratory syndrome coronavirus entry as a target of antiviral therapies.Antivir Ther. 2007;12(4 Pt B):639-50. Antivir Ther. 2007. PMID: 17944271 Review.
Cited by
-
GILT restricts the cellular entry mediated by the envelope glycoproteins of SARS-CoV, Ebola virus and Lassa fever virus.Emerg Microbes Infect. 2019;8(1):1511-1523. doi: 10.1080/22221751.2019.1677446. Emerg Microbes Infect. 2019. PMID: 31631785 Free PMC article.
-
Silibinin and SARS-CoV-2: Dual Targeting of Host Cytokine Storm and Virus Replication Machinery for Clinical Management of COVID-19 Patients.J Clin Med. 2020 Jun 7;9(6):1770. doi: 10.3390/jcm9061770. J Clin Med. 2020. PMID: 32517353 Free PMC article.
-
Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider.Front Immunol. 2020 Aug 18;11:2069. doi: 10.3389/fimmu.2020.02069. eCollection 2020. Front Immunol. 2020. PMID: 32973815 Free PMC article.
-
Iminosugars: A host-targeted approach to combat Flaviviridae infections.Antiviral Res. 2020 Dec;184:104881. doi: 10.1016/j.antiviral.2020.104881. Epub 2020 Aug 5. Antiviral Res. 2020. PMID: 32768411 Free PMC article. Review.
-
Exploring the Role of Glycans in the Interaction of SARS-CoV-2 RBD and Human Receptor ACE2.Viruses. 2021 May 17;13(5):927. doi: 10.3390/v13050927. Viruses. 2021. PMID: 34067878 Free PMC article.
References
-
- Meliopoulos VA, Andersen LE, Birrer KF, Simpson KJ, Lowenthal JW, Bean AG, Stambas J, Stewart CR, Tompkins SM, van Beusechem VW, Fraser I, Mhlanga M, Barichievy S, Smith Q, Leake D, Karpilow J, Buck A, Jona G, Tripp RA. 2012. Host gene targets for novel influenza therapies elucidated by high-throughput RNA interference screens. FASEB J 26:1372–1386. doi:10.1096/fj.11-193466. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

