Human cytomegalovirus resistance to deoxyribosylindole nucleosides maps to a transversion mutation in the terminase subunit-encoding gene UL89
- PMID: 25348532
- PMCID: PMC4291354
- DOI: 10.1128/AAC.03686-14
Human cytomegalovirus resistance to deoxyribosylindole nucleosides maps to a transversion mutation in the terminase subunit-encoding gene UL89
Abstract
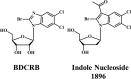
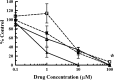
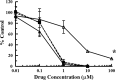
Human cytomegalovirus (HCMV) infection can cause severe illnesses, including encephalopathy and mental retardation, in immunocompromised and immunologically immature patients. Current pharmacotherapies for treating systemic HCMV infections include ganciclovir, cidofovir, and foscarnet. However, long-term administration of these agents can result in serious adverse effects (myelosuppression and/or nephrotoxicity) and the development of viral strains with reduced susceptibility to drugs. The deoxyribosylindole (indole) nucleosides demonstrate a 20-fold greater activity in vitro (the drug concentration at which 50% of the number of plaques was reduced with the presence of drug compared to the number in the absence of drug [EC50] = 0.34 μM) than ganciclovir (EC50 = 7.4 μM) without any observed increase in cytotoxicity. Based on structural similarity to the benzimidazole nucleosides, we hypothesize that the indole nucleosides target the HCMV terminase, an enzyme responsible for packaging viral DNA into capsids and cleaving the DNA into genome-length units. To test this hypothesis, an indole nucleoside-resistant HCMV strain was isolated, the open reading frames of the genes that encode the viral terminase were sequenced, and a G766C mutation in exon 1 of UL89 was identified; this mutation resulted in an E256Q change in the amino acid sequence of the corresponding protein. An HCMV wild-type strain, engineered with this mutation to confirm resistance, demonstrated an 18-fold decrease in susceptibility to the indole nucleosides (EC50 = 3.1 ± 0.7 μM) compared to that of wild-type virus (EC50 = 0.17 ± 0.04 μM). Interestingly, this mutation did not confer resistance to the benzimidazole nucleosides (EC50 for wild-type HCMV = 0.25 ± 0.04 μM, EC50 for HCMV pUL89 E256Q = 0.23 ± 0.04 μM). We conclude, therefore, that the G766C mutation that results in the E256Q substitution is unique for indole nucleoside resistance and distinct from previously discovered substitutions that confer both indole and benzimidazole nucleoside resistance (D344E and A355T).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Role of a mutation in human cytomegalovirus gene UL104 in resistance to benzimidazole ribonucleosides.J Virol. 2004 Jan;78(2):710-5. doi: 10.1128/jvi.78.2.710-715.2004. J Virol. 2004. PMID: 14694102 Free PMC article.
-
Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56.J Virol. 1998 Jun;72(6):4721-8. doi: 10.1128/JVI.72.6.4721-4728.1998. J Virol. 1998. PMID: 9573236 Free PMC article.
-
Comparison of Cytomegalovirus Terminase Gene Mutations Selected after Exposure to Three Distinct Inhibitor Compounds.Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01325-17. doi: 10.1128/AAC.01325-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 28827420 Free PMC article.
-
Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance.Antiviral Res. 2019 Mar;163:91-105. doi: 10.1016/j.antiviral.2019.01.011. Epub 2019 Jan 25. Antiviral Res. 2019. PMID: 30690043 Review.
-
Targeting the terminase: An important step forward in the treatment and prophylaxis of human cytomegalovirus infections.Antiviral Res. 2019 Jan;161:116-124. doi: 10.1016/j.antiviral.2018.11.005. Epub 2018 Nov 23. Antiviral Res. 2019. PMID: 30472161 Review.
Cited by
-
Progress in Treatment of Viral Infections in Children with Acute Lymphoblastic Leukemia.Oncol Rev. 2016 Jun 30;10(1):300. doi: 10.4081/oncol.2016.300. eCollection 2016 Apr 15. Oncol Rev. 2016. PMID: 27471584 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

