A hybrid cationic peptide composed of human β-defensin-1 and humanized θ-defensin sequences exhibits salt-resistant antimicrobial activity
- PMID: 25348533
- PMCID: PMC4291353
- DOI: 10.1128/AAC.03901-14
A hybrid cationic peptide composed of human β-defensin-1 and humanized θ-defensin sequences exhibits salt-resistant antimicrobial activity
Abstract
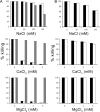
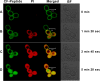
We have designed a hybrid peptide by combining sequences of human β-defensin-1 (HBD-1) and θ-defensin, in an attempt to generate a molecule that combines the diversity in structure and biological activity of two different peptides to yield a promising therapeutic candidate. HBD-1 was chosen as it is a natural defensin of humans that is constitutively expressed, but its antibacterial activity is considerably impaired by elevated ionic strength. θ-Defensins are expressed in human bone marrow as a pseudogene and are homologous to rhesus monkey circular minidefensins. Retrocyclins are synthetic human θ-defensins. The cyclic nature of the θ-defensin peptides makes them salt resistant, nonhemolytic, and virtually noncytotoxic in vitro. However, a nonhuman circular molecule developed for clinical use would be less viable than a linear molecule. In this study, we have fused the C-terminal region of HBD-1 to the nonapeptide sequence of a synthetic retrocyclin. Cyclization was achieved by joining the terminal ends of the hybrid peptide by a disulfide bridge. The hybrid peptide with or without the disulfide bridge exhibited enhanced antimicrobial activity against both Gram-negative and Gram-positive bacteria as well as against fungi, including clinical bacterial isolates from eye infections. The peptide retained activity in the presence of NaCl and serum and was nonhemolytic in vitro. Thus, the hybrid peptide generated holds potential as a new class of antibiotics.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria.BMC Microbiol. 2018 Jun 5;18(1):54. doi: 10.1186/s12866-018-1190-z. BMC Microbiol. 2018. PMID: 29871599 Free PMC article.
-
Biocidal activity of chicken defensin-9 against microbial pathogens.Biochem Cell Biol. 2016 Apr;94(2):176-87. doi: 10.1139/bcb-2015-0121. Epub 2015 Dec 16. Biochem Cell Biol. 2016. PMID: 26914652
-
Influence of disulfide bonds in human beta defensin-3 on its strain specific activity against Gram-negative bacteria.Biochim Biophys Acta Biomembr. 2020 Aug 1;1862(8):183273. doi: 10.1016/j.bbamem.2020.183273. Epub 2020 Mar 19. Biochim Biophys Acta Biomembr. 2020. PMID: 32171739
-
Theta-defensins: cyclic antimicrobial peptides produced by binary ligation of truncated alpha-defensins.Curr Protein Pept Sci. 2004 Oct;5(5):365-71. doi: 10.2174/1389203043379459. Curr Protein Pept Sci. 2004. PMID: 15544531 Review.
-
Cyclic Peptides as Novel Therapeutic Microbicides: Engineering of Human Defensin Mimetics.Molecules. 2017 Jul 20;22(7):1217. doi: 10.3390/molecules22071217. Molecules. 2017. PMID: 28726740 Free PMC article. Review.
Cited by
-
A Chimeric Cationic Peptide Composed of Human β-Defensin 3 and Human β-Defensin 4 Exhibits Improved Antibacterial Activity and Salt Resistance.Front Microbiol. 2021 May 7;12:663151. doi: 10.3389/fmicb.2021.663151. eCollection 2021. Front Microbiol. 2021. PMID: 34025617 Free PMC article.
-
Novel synthetic analogues of avian β-defensin-12: the role of charge, hydrophobicity, and disulfide bridges in biological functions.BMC Microbiol. 2017 Feb 23;17(1):43. doi: 10.1186/s12866-017-0959-9. BMC Microbiol. 2017. PMID: 28231771 Free PMC article.
-
Theta-Defensins to Counter COVID-19 as Furin Inhibitors: In Silico Efficiency Prediction and Novel Compound Design.Comput Math Methods Med. 2022 Feb 9;2022:9735626. doi: 10.1155/2022/9735626. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35154362 Free PMC article.
-
Perspectives for clinical use of engineered human host defense antimicrobial peptides.FEMS Microbiol Rev. 2017 May 1;41(3):323-342. doi: 10.1093/femsre/fux012. FEMS Microbiol Rev. 2017. PMID: 28521337 Free PMC article. Review.
-
Hybrids made from antimicrobial peptides with different mechanisms of action show enhanced membrane permeabilization.Biochim Biophys Acta Biomembr. 2019 Oct 1;1861(10):182980. doi: 10.1016/j.bbamem.2019.05.002. Epub 2019 May 5. Biochim Biophys Acta Biomembr. 2019. PMID: 31067436 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

