The R262K substitution combined with H51Y in HIV-1 subtype B integrase confers low-level resistance against dolutegravir
- PMID: 25348535
- PMCID: PMC4291366
- DOI: 10.1128/AAC.04274-14
The R262K substitution combined with H51Y in HIV-1 subtype B integrase confers low-level resistance against dolutegravir
Abstract
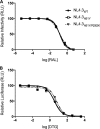
Clinical studies have shown that integrase strand transfer inhibitors (INSTIs) can be used effectively against HIV-1 infection. To date, no resistance substitution has been found in INSTI-naive patients treated with the new integrase inhibitor dolutegravir (DTG). In a recent selection study with DTG, using a virus bearing the H51Y substitution in integrase, the emergence of an R to K substitution at position 262 (R262K) was observed. We characterized this double mutant with respect to integrase strand transfer activity and susceptibility to DTG both biochemically and in tissue culture. We showed that the addition of R262K to H51Y decreased recombinant integrase strand transfer activity but improved integrase DNA-binding affinity, compared to wild-type or H51Y-containing enzymes. The defect in strand transfer activity did not translate into a decrease in HIV-1 infectivity. The combination of H51Y and R262K substitutions slightly decreased susceptibility to DTG (fold change = 1.87) in cell-based resistance assays. Although viral replication was not affected and enzyme efficiency was impaired by the addition of R262K to H51Y, there was an overall increase in the level of biochemical drug resistance against DTG. Our findings suggest that the R at position 262 plays an important role in DNA binding.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
The Combination of the R263K and T66I Resistance Substitutions in HIV-1 Integrase Is Incompatible with High-Level Viral Replication and the Development of High-Level Drug Resistance.J Virol. 2015 Nov;89(22):11269-74. doi: 10.1128/JVI.01881-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311878 Free PMC article.
-
Biochemical analysis of the role of G118R-linked dolutegravir drug resistance substitutions in HIV-1 integrase.Antimicrob Agents Chemother. 2013 Dec;57(12):6223-35. doi: 10.1128/AAC.01835-13. Epub 2013 Sep 30. Antimicrob Agents Chemother. 2013. PMID: 24080645 Free PMC article.
-
Dolutegravir-Selected HIV-1 Containing the N155H and R263K Resistance Substitutions Does Not Acquire Additional Compensatory Mutations under Drug Pressure That Lead to Higher-Level Resistance and Increased Replicative Capacity.J Virol. 2015 Oct;89(20):10482-8. doi: 10.1128/JVI.01725-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246578 Free PMC article.
-
Different Pathways Conferring Integrase Strand-Transfer Inhibitors Resistance.Viruses. 2022 Nov 22;14(12):2591. doi: 10.3390/v14122591. Viruses. 2022. PMID: 36560595 Free PMC article. Review.
-
Characterization and structural analysis of HIV-1 integrase conservation.AIDS Rev. 2009 Jan-Mar;11(1):17-29. AIDS Rev. 2009. PMID: 19290031 Review.
Cited by
-
Strain-specific effect on biphasic DNA binding by HIV-1 integrase.AIDS. 2019 Mar 1;33(3):588-592. doi: 10.1097/QAD.0000000000002078. AIDS. 2019. PMID: 30475264 Free PMC article.
-
HIV drug resistance against strand transfer integrase inhibitors.Retrovirology. 2017 Jun 5;14(1):36. doi: 10.1186/s12977-017-0360-7. Retrovirology. 2017. PMID: 28583191 Free PMC article. Review.
-
Different Pathways Leading to Integrase Inhibitors Resistance.Front Microbiol. 2017 Jan 11;7:2165. doi: 10.3389/fmicb.2016.02165. eCollection 2016. Front Microbiol. 2017. PMID: 28123383 Free PMC article. Review.
-
Tribute to Mark Wainberg.Retrovirology. 2017 Jun 28;14(1):38. doi: 10.1186/s12977-017-0361-6. Retrovirology. 2017. PMID: 28659190 Free PMC article. No abstract available.
-
In vitro activity of dolutegravir against wild-type and integrase inhibitor-resistant HIV-2.Retrovirology. 2015 Feb 5;12:10. doi: 10.1186/s12977-015-0146-8. Retrovirology. 2015. PMID: 25808007 Free PMC article.
References
-
- Hightower KE, Wang R, Deanda F, Johns BA, Weaver K, Shen Y, Tomberlin GH, Carter HL III, Broderick T, Sigethy S, Seki T, Kobayashi M, Underwood MR. 2011. Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother 55:4552–4559. doi:10.1128/AAC.00157-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

