Treatment for speech disorder in Friedreich ataxia and other hereditary ataxia syndromes
- PMID: 25348587
- PMCID: PMC11214034
- DOI: 10.1002/14651858.CD008953.pub2
Treatment for speech disorder in Friedreich ataxia and other hereditary ataxia syndromes
Abstract
Background: Hereditary ataxia syndromes can result in significant speech impairment, a symptom thought to be responsive to treatment. The type of speech impairment most commonly reported in hereditary ataxias is dysarthria. Dysarthria is a collective term referring to a group of movement disorders affecting the muscular control of speech. Dysarthria affects the ability of individuals to communicate and to participate in society. This in turn reduces quality of life. Given the harmful impact of speech disorder on a person's functioning, treatment of speech impairment in these conditions is important and evidence-based interventions are needed.
Objectives: To assess the effects of interventions for speech disorder in adults and children with Friedreich ataxia and other hereditary ataxias.
Search methods: On 14 October 2013, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL, MEDLINE, EMBASE, CINAHL Plus, PsycINFO, Education Resources Information Center (ERIC), Linguistics and Language Behavior Abstracts (LLBA), Dissertation Abstracts and trials registries. We checked all references in the identified trials to identify any additional published data.
Selection criteria: We considered for inclusion randomised controlled trials (RCTs) or quasi-RCTs that compared treatments for hereditary ataxias with no treatment, placebo or another treatment or combination of treatments, where investigators measured speech production.
Data collection and analysis: Two review authors independently selected trials for inclusion, extracted data and assessed the risk of bias of included studies using the standard methodological procedures expected by The Cochrane Collaboration. The review authors collected information on adverse effects from included studies. We did not conduct a meta-analysis as no two studies utilised the same assessment procedures within the same treatment.
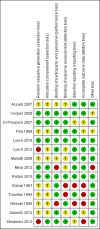
Main results: Fourteen clinical trials, involving 721 participants, met the criteria for inclusion in the review. Thirteen studies compared a pharmaceutical treatment with placebo (or a low dose of the intervention), in heterogenous groups of degenerative cerebellar ataxias. Three compounds were studied in two trials each: a levorotatory form of 5-hydroxytryptophan (L-5HT), idebenone and thyrotropin-releasing hormone tartrate (TRH-T); each of the other compounds (riluzole, varenicline, buspirone, betamethasone, coenzyme Q10 with vitamin E, α-tocopheryl quinone and erythropoietin) were studied in one trial. The 14th trial, involving a mixed group of participants with spinocerebellar ataxia, compared the effectiveness of nonspecific physiotherapy and occupational therapy within an inpatient hospital setting to no treatment. No studies utilised traditional speech therapies. We defined the primary outcome measure in this review as the percentage change (improvement) in overall speech production immediately following completion of the intervention or later, measured by any validated speech assessment tool. None of the trials included speech as a primary outcome or examined speech using any validated speech assessment tool. Eleven studies reported speech outcomes derived from a subscale embedded within disease rating scales. The remaining three studies used alternative assessments to measure speech, including mean time to produce a standard sentence, a subjective rating of speech on a 14-point analogue scale, patient-reported assessment of the impact of dysarthria on activities of daily living and acoustic measures of syllable length. One study measured speech both subjectively as part of a disease rating scale and with further measures of speech timing. Three studies utilised the Short Form-36 Health Survey (SF-36) and one used the Child Health Questionnaire as measures of general quality of life. A further study utilised the Functional Independence Measure to assess functional health.Five studies reported statistically significant improvement on an overall disease rating scale in which a speech subscale was included. Only three of those studies provided specific data on speech performance; all were comparisons with placebo. Improvements in overall disease severity were observed with α-tocopheryl quinone; however, no significant changes were found on the speech subscale in a group of individuals with Friedreich ataxia. A statistically significant improvement in speech according to a speech disorders subscale was observed with betamethasone. Riluzole was found to have a statistically significant effect on speech in a group of participants with mixed hereditary, sporadic and unknown origin ataxias. No significant differences were observed between treatment and placebo in any other pharmaceutical study. A statistically significant improvement in functional independence occurred at the end of the treatment period in the rehabilitation study compared to the delayed treatment group but these effects were not present 12 to 24 weeks after treatment. Of the four studies that assessed quality of life, none found a significant effect. A variety of minor adverse events were reported for the 13 pharmaceutical therapies, including gastrointestinal side effects and nausea. Serious adverse effects were reported in two participants in one of the L-5HT trials (participants discontinued due to gastrointestinal effects), and in four participants (three taking idebenone, one taking placebo) in the idebenone studies. Serious adverse events with idebenone were gastrointestinal side effects and, in people with a previous history of these events, chest pain and idiopathic thrombocytopenic purpura. The rehabilitation study did not report any adverse events.We considered six studies to be at high risk of bias in some respect. We suspected inadequate blinding of participants or assessors in four studies and poor randomisation in a further two studies. There was a high risk of reporting bias in two studies and attrition bias in four studies. Only one study had a low risk of bias across all criteria. Taken together with other limitations of the studies relating to the validity of the measurement scales used, we downgraded the quality of the evidence for many of the outcomes to low or very low.
Authors' conclusions: There is insufficient and low or very low quality evidence from either RCTs or observational studies to determine the effectiveness of any treatment for speech disorder in any of the hereditary ataxia syndromes.
Conflict of interest statement
AV: no known conflicts of interest to declare. JF: no known conflicts of interest to declare. MLP: no known conflicts of interest to declare.
Figures
Update of
References
References to studies included in this review
Assadi 2007 {published data only}
-
- Assadi M, Campellone JV, Janson CG, Veloski JJ, Schwartzman RJ, Leone P. Treatment of spinocerebellar ataxia with buspirone. Journal of the Neurological Sciences 2007;260(1‐2):143‐6. [PUBMED: 17512011] - PubMed
Cooper 2008 {published data only}
-
- Cooper JM, Korlipara LVP, Hart PE, Bradley JL, Schapira AHV. Coenzyme Q10 and vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. European Journal of Neurology 2008;15(12):1371‐9. [PUBMED: 19049556] - PubMed
Di Prospero 2007 {published data only (unpublished sought but not used)}
-
- Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high‐dose idebenone in patients with Friedreich's ataxia: a randomised, placebo‐controlled trial. Lancet Neurology 2007;6(10):878‐86. [PUBMED: 17826341] - PubMed
Filla 1988 {published data only (unpublished sought but not used)}
-
- Filla A, Michele G, Martino L, Mengano A, Iorio L, Maggio MA, et al. Chronic experimentation with TRH administered intramuscularly in spinocerebellar degeneration. Double‐blind cross‐over study in 30 subjects. In: Agnoli A, Delwaide PJ, Prange AJ, Scapagnini U editor(s). Protirelin Tartrate (TRH‐T): Pharmacological and Clinical Studies: Recent Advances and Perspectives. London: John Libbey, 1988:199‐209.
-
- Filla A, Michele G, Martino L, Mengano A, Iorio L, Maggio MA, et al. Chronic experimentation with TRH administered intramuscularly in spinocerebellar degeneration. Double‐blind cross‐over study in 30 subjects. Rivista di Neurologia 1989;59(2):83‐8. [PUBMED: 2505370] - PubMed
Lynch 2010 {published data only (unpublished sought but not used)}
-
- Lynch DR, Perlman SL, Meier T. A phase 3, double‐blind, placebo‐controlled trial of idebenone in Friedreich ataxia. Archives of Neurology 2010;67(8):941‐7. [PUBMED: 20697044] - PubMed
Lynch 2012 {published data only}
-
- Lynch DR, Willi SM, Wilson RB, Cotticelli MG, Brigatti KW, Deutsch EC, et al. A0001 in Friedreich ataxia: biochemical characterization and effects in a clinical trial. Movement Disorders 2012;27(8):1026‐33. [PUBMED: 22744651] - PubMed
Mariotti 2009 {published data only}
-
- Mariotti C, Fancellu R, Caldarazzo S, Nanetti L, Bella D, Plumari M, et al. Erythropoietin in Friedreich ataxia: no effect on frataxin in a randomized controlled trial. Movement Disorders 2012;27(3):446‐9. - PubMed
Miyai 2012 {published data only}
-
- Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, Yagura H, et al. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabilitation and Neural Repair 2012;26(5):515‐22. [PUBMED: 22140200] - PubMed
Ristori 2010 {published data only}
-
- Ristori G, Romano S, Visconti A, Cannoni S, Spadaro M, Frontali M, et al. Riluzole in cerebellar ataxia: a randomized, double‐blind, placebo‐controlled pilot trial. Neurology 2010;74(10):839‐45. [PUBMED: 20211908] - PubMed
Sobue 1983 {published data only (unpublished sought but not used)}
-
- Sobue I, Takayanagi T, Nakanishi T, Tsubaki T, Uono M, Kinoshita M, et al. Controlled trial of thyrotropin releasing hormone tartrate in ataxia of spinocerebellar degenerations. Journal of the Neurological Sciences 1983;61(2):235‐48. [PUBMED: 6417282] - PubMed
Trouillas 1995 {published data only (unpublished sought but not used)}
-
- Trouillas P, Serratrice G, Laplane D, Rascol A, Augustin P, Barroche G, et al. Levorotatory form of 5‐hydroxytryptophan in Friedreich's ataxia. Archives of Neurology 1995;52(5):456‐60. [PUBMED: 7733839] - PubMed
Wessel 1995 {published data only}
-
- Wessel K, Hermsdörfer J, Deger K, Herzog T, Huss GP, Kömpf D, et al. Double‐blind crossover study with levorotatory form of hydroxytryptophan in patients with degenerative cerebellar diseases. Archives of Neurology 1995;52(5):451‐5. [PUBMED: 7733838] - PubMed
Zannolli 2012 {published data only}
-
- Zannolli R, Buoni S, Betti G, Salvucci S, Plebani A, Soresina A, et al. A randomized trial of oral betamethasone to reduce ataxia symptoms in ataxia telangiectasia. Movement Disorders 2012;27(10):1312‐6. [PUBMED: 22927201] - PubMed
Zesiewicz 2012 {published data only}
-
- Zesiewicz TA, Greenstein PE, Sullivan KL, Wecker L, Miller A, Jahan I, et al. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology 2012;78(8):545‐50. [PUBMED: 22323747] - PubMed
References to studies excluded from this review
Arnold 2006 {published data only}
-
- Arnold P, Boulat O, Malre R, Kuntzer T. Expanding view of phenotype and oxidative stress in Friedreich's ataxia patients with and without idebenone. Schweizer Archiv für Neurologie und Psychiatrie 2006;157(4):169‐76.
Artuch 2002 {published data only}
-
- Artuch R, Aracil A, Mas A, Colomé C, Rissech M, Monrós E, et al. Friedreich's ataxia: idebenone treatment in early stage patients. Neuropediatrics 2002;33(4):190‐3. - PubMed
Artuch 2006 {published data only}
-
- Artuch R, Brea‐Calvo G, Briones P, Aracil A, Galván M, Espinós C, et al. Cerebellar ataxia with coenzyme Q10 deficiency: diagnosis and follow‐up after coenzyme Q10 supplementation. Journal of the Neurological Sciences 2006;246(1‐2):153‐8. - PubMed
Boesch 2007 {published data only}
-
- Boesch S, Sturm B, Hering S, Goldenberg H, Poewe W, Scheiber‐Mojdehkar B. Friedreich's ataxia: clinical pilot trial with recombinant human erythropoietin. Annals of Neurology 2007;62(5):521‐4. - PubMed
Boesch 2008 {published data only}
-
- Boesch S, Sturm B, Hering S, Scheiber‐Mojdehkar B, Steinkellner H, Goldenberg H, et al. Neurological effects of recombinant human erythropoietin in Friedreich's ataxia: a clinical pilot trial. Movement Disorders 2008;23(13):1940‐4. - PubMed
Bonnan 2008 {published data only}
-
- Bonnan M, Cabre P, Olindo S, Signate A, Saint‐Vil M, Smadja D. Steroid treatment in four cases of anti‐GAD cerebellar ataxia [Traitement des ataxies cérébelleuses à anticorps anti‐GAD par cures séquentielles de corticoïdes]. Revue Neurologique 2008;164(5):427‐33. - PubMed
Botez 1996 {published data only}
Botez 1997 {published data only}
-
- Botez MI, Mayer P, Bellemare F, Couture J. Can we treat respiratory failure in Friedreich ataxia?. Archives of Neurology 1997;54(8):1030‐3. - PubMed
Broccoletti 2008 {published data only}
-
- Broccoletti T, Giudice E, Amorosi S, Russo I, Bonito M, Imperati F, et al. Steroid‐induced improvement of neurological signs in ataxia‐telangiectasia patients. European Journal of Neurology 2008;15(3):223‐8. - PubMed
Ershova 2007 {published data only}
-
- Ershova MV, Illarioshkin SN, Sukhorukov VS. The use of noben for correction of mitochondrial disorders in Friedrich's disease. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2007;107(9):32‐7. - PubMed
Heo 2008 {published data only}
-
- Heo JH, Lee ST, Chu K, Kim M. The efficacy of combined estrogen and buspirone treatment in olivopontocerebellar atrophy. Journal of the Neurological Sciences 2008;271(1‐2):87‐90. - PubMed
Ilg 2012 {published data only}
-
- Ilg W, Schatton C, Schicks J, Giese MA, Schöls L, Synofzik M. Video game–based coordinative training improves ataxia in children with degenerative ataxia. Neurology 2012;79(20):2056‐60. - PubMed
Lagedrost 2011 {published data only}
-
- Lagedrost SJ, Sutton MS, Cohen MS, Satou GM, Kaufman BD, Perlman SL, et al. Idebenone in Friedreich ataxia cardiomyopathy—results from a 6‐month phase III study (IONIA). American Heart Journal 2011;161(3):639‐45. [10.1016/j.ahj.2010.10.038] - PubMed
Meier 2012 {published data only}
-
- Meier T, Perlman SL, Rummey C, Coppard NJ, Lynch DR. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich's ataxia: data from a 6‐month controlled study followed by a 12‐month open‐label extension study. Journal of Neurology 2012;259(2):284‐91. [10.1007/s00415‐011‐6174‐y] - PubMed
Melancon 1982 {published data only}
-
- Melancon SB, Vanasse M, Geoffroy G, Barabe L, Proulx A, Fontaine G, et al. Oral lecithin and linoleic acid in Friedreich's ataxia: II. Clinical results. Canadian Journal of Neurological Sciences 1982;9(2):155‐64. - PubMed
Nakamura 2009 {published data only}
Ogawa 2003 {published data only}
-
- Ogawa M, Shigeto H, Yamamoto T, Oya Y, Wada K, Nishikawa T, et al. D‐cycloserine for the treatment of ataxia in spinocerebellar degeneration. Journal of the Neurological Sciences 2003;210(1‐2):53‐6. - PubMed
Pineda 2008 {published data only}
Shimizu 1999 {published data only}
-
- Shimizu H, Tsuda T, Shiga Y, Miyazawa K, Onodera Y, Matsuzaki M, et al. Therapeutic efficacy of transcranial magnetic stimulation for hereditary spinocerebellar degeneration. Tohoku Journal of Experimental Medicine 1999;189(3):203‐11. - PubMed
Sobue 1980 {published data only}
-
- Sobue I, Yamamoto H, Konagaya M, Iida M, Takayanagi T. Effect of thyrotropin‐releasing hormone on ataxia of spinocerebellar degeneration. Lancet 1980;315(8165):418‐9. - PubMed
Strupp 2011 {published data only}
Trouillas 1984 {published data only}
-
- Trouillas P. Regression of cerebellar syndrome with long‐term administration of 5‐HTP or the combination 5‐HTP‐benserazide. Italian Journal of Neurological Sciences 1984;5(3):253‐66. - PubMed
Trouillas 1997 {published data only}
-
- Trouillas P, Xie J, Adeleine P, Michel D, Vighetto A, Honnorat J, et al. Buspirone, a 5‐hydroxytryptamine1A agonist, is active in cerebellar ataxia. Results of a double‐blind drug placebo study in patients with cerebellar cortical atrophy. Archives of Neurology 1997;54(6):749‐52. - PubMed
Velasco‐Sanchez 2011 {published data only}
Yabe 1999 {published data only (unpublished sought but not used)}
-
- Yabe I, Sasaki H, Yamashita I, Takei A, Fukazawa T, Hamada T, et al. A clinical trial of acetazolamide for SCA6. Rinsho Shinkeigaku (Clinical Neurology) 1999;39(8):793‐9. - PubMed
References to ongoing studies
EUCTR 2009‐016317‐20‐IT {published data only (unpublished sought but not used)}
-
- EUCTR 2009‐016317‐20‐IT. Pilot study to assess safety and tolerability of lithium on spinocerebellar ataxia of type 2 ‐ lithium in SCA2. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐016317‐20‐IT (accessed 21 March 2014).
EUCTR 2012‐005312‐26‐DE {unpublished data only}
-
- EUCTR 2012‐005312‐26‐DE. Sustained release 4‐aminopyridine (Fampyra®) in cerebellar gait disorder. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐005312‐26‐DE (accessed 21 March 2014).
Schulz 2009 {published data only}
-
- Schulz J, Holder G, Meier T, on behalf of the MICONOS Study Investigators. 12‐month European phase III clinical study of SNT‐MC17/idebenone in the treatment of Friedreich’s ataxia: baseline neurology data and interim safety results. Journal of Neurology 2009;256 Suppl 2:S153.
Additional references
Campuzano 1996
-
- Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996;271(5254):1423‐7. - PubMed
Collie 2003
-
- Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test‐retest intervals. Journal of the International Neuropsychological Society 2003;9(3):419‐28. - PubMed
Delatycki 1999
-
- Delatycki MB, Paris DB, Gardner RJ, Nicholson GA, Nassif N, Storey E, et al. Clinical and genetic study of Friedreich ataxia in an Australian population. American Journal of Medical Genetics 1999;87(2):168‐74. - PubMed
Delatycki 2000
Duffy 2013
-
- Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. 3rd Edition. St Louis, MO: Elsevier Mosby, 2013.
Dürr 1996
-
- Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. New England Journal of Medicine 1996;335(16):1169‐75. - PubMed
Filla 2012
-
- Filla A, Sacca F, Michele G. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology 2012;78(19):1538. - PubMed
Folker 2010
-
- Folker JE, Murdoch BE, Cahill LM, Delatycki MB, Corben LA, Vogel AP. Dysarthria in Friedreich’s ataxia: a perceptual analysis. Folia Phoniatrica et Logopaedia 2010;62(3):97‐103. - PubMed
Gibilisco 2013
GRADEpro 2008 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Harding 1981
-
- Harding AE. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 1981;104(3):589‐620. - PubMed
Hartelius 2007
-
- Hartelius L, Elmberg M, Holm R, Lövberg AS, Nikolaidis S. Living with dysarthria: evaluation of a self‐report questionnaire. Folia Phoniatrica et Logopaedica 2007;60(1):11‐9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kearney 2012
Morgan 2008
Mundt 2012
Orr 1993
-
- Orr HT, Chung MY, Banfi S, Kwiatkowski TJ Jr, Servadio A, Beaudet AL. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type‐1. Nature Genetics 1993;4(3):221‐6. - PubMed
Ramig 2001
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosen 2012
-
- Rosen KM, Folker JE, Vogel AP, Corben LA, Murdoch BE, Delatycki MB. Longitudinal change in dysarthria associated with Friedreich ataxia: a potential clinical endpoint. Journal of Neurology 2012;259(11):2471‐7. - PubMed
Schalling 2007
-
- Schalling E, Hammarberg B, Hartelius L. Perceptual and acoustic analysis of speech in individuals with spinocerebellar ataxia (SCA). Logopedics, Phoniatrics, Vocology 2007;32(1):31‐46. - PubMed
Schöls 1997
-
- Schöls L, Amoiridis G, Przuntek H, Frank G, Epplen JT, Epplen C. Friedreich's ataxia. Revision of the phenotype according to molecular genetics. Brain 1997;120(Pt 12):2131‐40. - PubMed
Vogel 2010
Vogel 2010b
-
- Vogel AP, Fletcher J, Maruff P. Acoustic analysis of the effect of sustained wakefulness on speech. Journal of the Acoustical Society of America 2010;128(6):3747‐56. - PubMed
Vogel 2011
-
- Vogel AP, Fletcher J, Snyder PJ, Fredrickson A, Maruff P. Reliability, stability, and sensitivity to change and impairment in acoustic measures of timing and frequency. Journal of Voice 2011;25(2):137‐49. - PubMed
Vogel 2011b
Vogel 2012
-
- Vogel AP, Shirbin C, Churchyard AJ, Stout JC. Speech acoustic markers of early stage and prodromal Huntington's disease: a marker of disease onset?. Neuropsychologia 2012;50(14):3273‐8. - PubMed
Vogel 2014
-
- Vogel AP, Maruff P. Monitoring change requires a re‐think of assessment practices in voice and speech. Logopedics, Phoniatrics, Vocology 2014;39(2):56‐61. [DOI: ] - PubMed
WHO 2001
-
- World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization 2001.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous