Intestinal alkaline phosphatase deficiency leads to lipopolysaccharide desensitization and faster weight gain
- PMID: 25348635
- PMCID: PMC4288875
- DOI: 10.1128/IAI.02520-14
Intestinal alkaline phosphatase deficiency leads to lipopolysaccharide desensitization and faster weight gain
Abstract
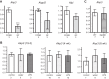
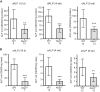
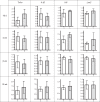
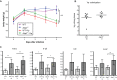
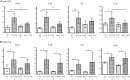
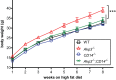
Animals develop in the presence of complex microbial communities, and early host responses to these microbes can influence key aspects of development, such as maturation of the immune system, in ways that impact adult physiology. We previously showed that the zebrafish intestinal alkaline phosphatase (ALPI) gene alpi.1 was induced by Gram-negative bacterium-derived lipopolysaccharide (LPS), a process dependent on myeloid differentiation primary response gene 88 (MYD88), and functioned to detoxify LPS and prevent excessive host inflammatory responses to commensal microbiota in the newly colonized intestine. In the present study, we examined whether the regulation and function of ALPI were conserved in mammals. We found that among the mouse ALPI genes, Akp3 was specifically upregulated by the microbiota, but through a mechanism independent of LPS or MYD88. We showed that disruption of Akp3 did not significantly affect intestinal inflammatory responses to commensal microbiota or animal susceptibility to Yersinia pseudotuberculosis infection. However, we found that Akp3(-/-) mice acquired LPS tolerance during postweaning development, suggesting that Akp3 plays an important role in immune education. Finally, we demonstrated that inhibiting LPS sensing with a mutation in CD14 abrogated the accelerated weight gain in Akp3(-/-) mice receiving a high-fat diet, suggesting that the weight gain is caused by excessive LPS in Akp3(-/-) mice.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- McComb RB, Bowers GN Jr, Posen S. 1979. Alkaline phosphatase. Plenum Press, Inc, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

