Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice
- PMID: 25348938
- PMCID: PMC4444057
- DOI: 10.1002/jbmr.2389
Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice
Abstract
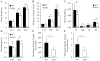
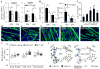
It is well-established that muscle forces are necessary for bone development as well as proper bone modeling and remodeling. Recent work has also suggested that bone acts as an endocrine organ that can influence the development of other organs. Connexin43 (Cx43), a gap junction protein that transduces mechanical signals, is an important determinant of cortical bone modeling. Using an osteoblast/osteocyte-specific ablation of the Cx43 gene (Gja1) driven by the 2.3-kb Col1 α1 promoter (cKO) in the mouse, in this study we confirmed reduced cortical bone thickness and density with expanded bone marrow cavity in the cKO humerus. Surprisingly, Gja1 deletion in bone cells also affected skeletal muscle development, resulting in lower fast muscle weight, grip strength, and maximum absolute and specific tetanic forces (60% to 80%, 85%, and 50%, respectively, of WT mice). The normally fast twitch extensor digitorum longus (EDL) muscle exhibited increased slow twitch fibers in cKO mice. These muscle defects were accompanied by a 40% to 60% reduction in mRNA abundance for genes encoding osteocalcin in the humerus, relative to WT mice. Accordingly, both carboxylated and undercarboxylated isoforms of osteocalcin were reduced by over 30% in the circulation of cKO mice. Moreover, the active, undercarboxylated isoform of osteocalcin (glu-OC) promoted myotube formation in C2C12 myoblast cultures, and glu-OC injections to cKO mice rescued EDL muscle cross-sectional area and grip strength in vivo. These findings demonstrate that Cx43 in osteoblasts/osteocytes indirectly modulates skeletal muscle growth and function, potentially via an endocrine effect of glu-OC.
Keywords: BONE-MUSCLE INTERACTIONS; CONNEXIN43; GAP JUNCTION; OSTEOCALCIN.
© 2014 American Society for Bone and Mineral Research.
Figures

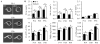
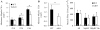
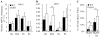
Comment in
-
Does defective bone lead to defective muscle?J Bone Miner Res. 2015 Apr;30(4):593-5. doi: 10.1002/jbmr.2491. J Bone Miner Res. 2015. PMID: 25727709 No abstract available.
References
-
- Wolff J. In: The Law of Bone Remodeling. Maquet P, Furlong R, translators. Berlin Heidelberg New York: Springer; 1986. (translation of the German 1892 edition)
-
- Shwartz Y, Blitz E, Zelzer E. One load to rule them all: mechanical control of the musculoskeletal system in development and aging. Differentiation. 2013;86:104–11. - PubMed
-
- Rolfe R, Roddy K, Murphy P. Mechanical regulation of skeletal development. Curr Osteoporos Rep. 2013;11:107–16. - PubMed
-
- Ward KA, Caulton JM, Adams JE, Mughal MZ. Perspective: cerebral palsy as a model of bone development in the absence of postnatal mechanical factors. J Musculoskelet Neuronal Interact. 2006;6:154–9. - PubMed
-
- Pearl ML, Edgerton BW, Kon DS, Darakjian AB, Kosco AE, Kazimiroff PB, et al. Comparison of arthroscopic findings with magnetic resonance imaging and arthrography in children with glenohumeral deformities secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 2003;85-A:890–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

