gigantea suppresses immutans variegation by interactions with cytokinin and gibberellin signaling pathways
- PMID: 25349324
- PMCID: PMC4256849
- DOI: 10.1104/pp.114.250647
gigantea suppresses immutans variegation by interactions with cytokinin and gibberellin signaling pathways
Abstract
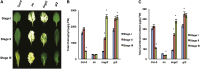
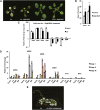
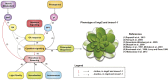
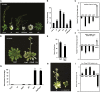
The immutans (im) variegation mutant of Arabidopsis (Arabidopsis thaliana) is an ideal model to gain insight into factors that control chloroplast biogenesis. im defines the gene for PTOX, a plastoquinol terminal oxidase that participates in the control of thylakoid redox. Here, we report that the im defect can be suppressed during the late stages of plant development by gigantea (gi2), which defines the gene for GI, a central component of the circadian clock that plays a poorly understood role in diverse plant developmental processes. imgi2 mutants are late flowering and display other well-known phenotypes associated with gi2, such as starch accumulation and resistance to oxidative stress. We show that the restoration of chloroplast biogenesis in imgi2 is caused by a development-specific derepression of cytokinin signaling that involves cross talk with signaling pathways mediated by gibberellin (GA) and SPINDLY (SPY), a GA response inhibitor. Suppression of the plastid defect in imgi2 is likely caused by a relaxation of excitation pressures in developing plastids by factors contributed by gi2, including enhanced rates of photosynthesis and increased resistance to oxidative stress. Interestingly, the suppression phenotype of imgi can be mimicked by crossing im with the starch accumulation mutant, starch excess1 (sex1), perhaps because sex1 utilizes pathways similar to gi. We conclude that our studies provide a direct genetic linkage between GI and chloroplast biogenesis, and we construct a model of interactions between signaling pathways mediated by gi, GA, SPY, cytokinins, and sex1 that are required for chloroplast biogenesis.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures






References
-
- Albrecht M, Klein A, Hugueney P, Sandmann G, Kuntz M (1995) Molecular cloning and functional expression in E. coli of a novel plant enzyme mediating ζ-carotene desaturation. FEBS Lett 372: 199–202 - PubMed
-
- Aluru MR, Stessman DJ, Spalding MH, Rodermel SR (2007) Alterations in photosynthesis in Arabidopsis lacking IMMUTANS, a chloroplast terminal oxidase. Photosynth Res 91: 11–23 - PubMed
-
- Baerr JN, Thomas JD, Taylor BG, Rodermel SR, Gray GR (2005) Differential photosynthetic compensatory mechanisms exist in the immutans mutant of Arabidopsis thaliana. Physiol Plant 124: 390–402
-
- Cao S, Jiang S, Zhang R (2006) The role of GIGANTEA gene in mediating the oxidative stress response and in Arabidopsis. Plant Growth Regul 48: 261–270
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

